Cell Motility and the Cytoskeleton-
Microtubules CS4
Microtubules are ideally suited as tracks to deliver
vesicles and organelles to distal cellular regions. They are rigid and so comparatively straight, they are polar with
the plus end almost invariably pointing to the periphery. Intracellular transport and motility is
mediated by MTs, but there is no direct involvement in crawling cell locomotion. Although cells with a highly developed
microtubule network such as fibroblasts and newt eosinophils become depolarised
and loose speed and directionality upon disruption of microtubules, it is
likely that microtubules disruption causes concomitant changes in the
microfilament system. Cells that are
naturally devoid of cytoplasmic microtubules (e.g. Naegleria) are capable of efficient and rapid locomotion. MTs
are abundant and crucial components of the flagella which drives the swimming
locomotion of eukaryotic cells. A
multitude of MT-based motors drives the chromosome reorganisation at mitosis
and meiosis. Two main groups propel
vesicles and organelles along MT in the cytoplasm; cytoplasmic dynein and a
more diverse group, the kinesins
Cytoplasmic Dynein
Cytoplasmic dynein is also known as MAP1C, it is a large two
headed ATP-ase which is minus end directed. Some dyneins from the flagella of Chlamydomonas and Tetrahymena
have three heads, but most other flagellar dyneins have just the two. Cytoplasmic dynein moves vesicles at around
0.3 µm/sec in vitro, is unaffected by
NEM and Vanadate and can utilise ATP, GTP or ITP for force production.
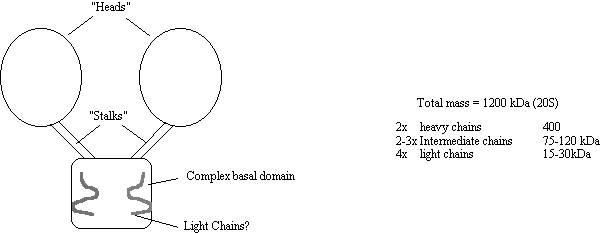
Figure 18
The Kinesins
The kinesins are a large group of microtubule based motor
proteins which are formed from multi-component complexes. The first kinesin was identified in the axon
in 1985 but several other kinesin related proteins (KRP) have come to
light. Three kinesin subfamilies have
been implicated in the plus end directed movement of vesicles and
organelles. All three share a similar
kinesin “head” domain but are otherwise quite different. Although they all seem to carry vesicles it
is suspected that each carries a specific type of vesicle. Kinesin moves vesicles at around 1.25
µm/sec, is inhibited by NEM and Vanadate and can only utilise ATP for force
production
___________________________________________________________________________________________________
Protein direction Mol.Wt (kDa) Speed Cargo
of
heavy chain (µm/min)
___________________________________________________________________________________________________
Kinesin plus 110 (heterotetrameric) 30-54 Vesicles
Unc-104
(KIF1A) plus 192 (monomeric) 72 precursor vesicles
(KIF1B) plus 130 (monomeric) 40 Mitochondria
Kinesin
II (KRP85/95) plus 79
(heterotrimer) 24 Vesicles
Many other kinesin related motor proteins have been
discovered which are involved in the spindle pole formation and
karyokinesis. These have largely been
identified at the gene level by genetic screen and so not much is known about
their biochemical properties. However,
one of these, NCD is a kinesin related protein which drives minus end movement!
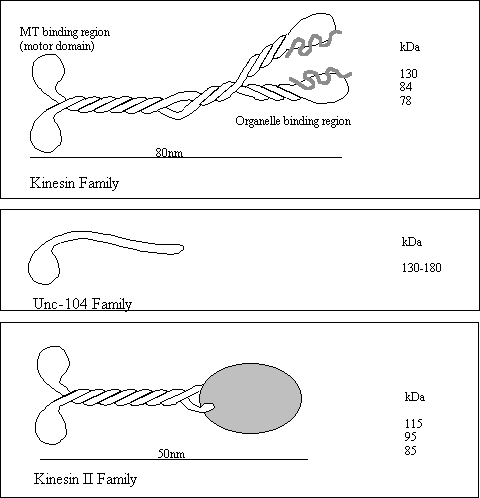
Figure 19
Control of Dynein
and Kinesin motor activity.
In most cell types, MTs have their plus ends facing the
plasma membrane and the minus end associated with a MTOC deep in side the
cell. Kinesins will therefore generally
move organelles toward the plasma-membrane and dyneins take them towards the
nucleus. A spectacular example of the
is the movement of pigment granules along the MT in the chromatophore from the skin of a variety of fish and
amphibians. Chromophores are stellate
flattened cell types which contain a multitude of dense light absorbing
granules. When the granules are packed
in the centre of the cell, the skin appears light, but darkens when the pigment
granules are dispersed throughout the cell.
This affords the animal protective camouflage. Granule movement requires microtubules. Dispersion depends on cAMP or IP3/diacylglycerol, the activity of
PKA or PKC, and kinesin. Aggregation
depends on phosphatase activity and it thought to be dynein dependent, although
this step is very rapid at 5µm/sec and dynein driven motility is only 0.3
µm/sec in vitro. Cellular cofactors absent from the in vitro studies may account for this
difference.
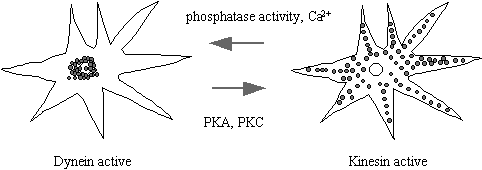
Figure 20
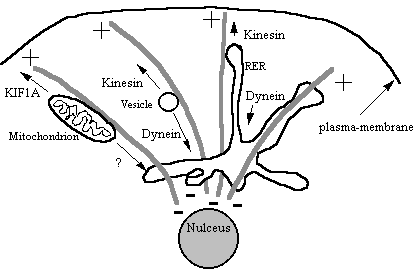
Figure 21
Other membranous organelles such as the Gogli, RER,
lysosomes and mitochondria are moved around the cell on microtubules. Many of these share the same motors so it is
difficult to see how the cell switches on motility of one particular organelle
and not others. Perhaps motor receptors
are the key (see later), localised phosphorylation of motor protein complexes
is perhaps another controlling mechanism.
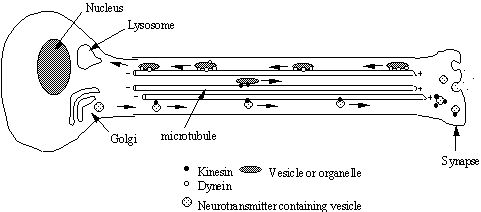
Figure 22
Transport of material along the nerve axon. Materials such as neurotransmitter peptides
are synthesised in the cell body and sequestered in vesicles at the golgi. These vesicles are then transported down the
axon towards the synapse by kinesin motor proteins. This distance may be yards in the case of a giraffe sciatic
nerve! Other materials are transported
from the synapse to the cell body by dynein motors.
Co-operation
between Microtubules and Microfilament systems in vesicular transport
In many cases there appears to be considerable overlap in
the vesicular motility driven by the MT and MF systems. Mitochondria are moved along MTs by KIF1B,
and along MFs at 1.4µm/min by an unidentified myosin I-like activity (at least
in yeast). Also yeast cell with
disabled kinesin are rescued by overexpression of myosin I!
Are there specific
Kinesin and Dynein receptors on cargo membranes?
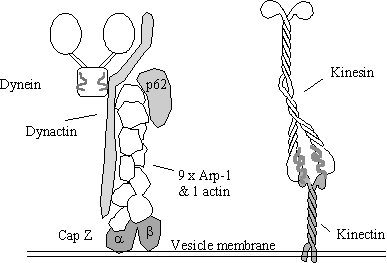
Recently a putative receptor “kinectin” has been identified
by passing detergent-solubilized microsomal membranes over a kinesin affinity
column. Kinectin has a molecular weight
of 160 kDa and the cloned cDNA reveals that the protein has a hydrophobic
N-terminus and a high probability of forming coiled-coil Kinectin has numerous putative phosphorylation
sites and is known to be phosphorylated on serine, however there has been no
correlation between the state of phosphorylation and kinesin binding activity
to date. Kinectin is most abundant on
the ER membranes, and is not thought to bind dynein. It is very likely that receptors other than kinectin exist in or
on other membranes. A possible
candidate for a dynein receptor is “dynactin”, a 150-170 kDa protein.
Why are organelles
arranged and moved along MTs and MFs?
When a cell divides in two each half requires the correct
amount of organelle activity to be included in its share. Organelles such as mitochondria are only
made from existing mitochondria and cannot be synthesised de novo. If a daughter cell finds itself without
enough mitochondria, it may not be capable of producing all its energy
requirements and so may die before being able to grow more mitochondria. The microfilament and microtubule system is
shared very equitable between daughter cells because they constitute the very
apparatus which separates them, thus if organelles are arranged on these
structures this will guarantee an equitable disruption of organelles between
daughter cells. Other cell type have
got around this problem using different means.
A large amoeba Pelomyxa palustris
does not have mitochondria, but does have symbiotic bacteria which attach
themselves to the nuclear membrane during cytokinesis. (This works because unlike higher eukaryotes
the nuclear membrane does not break down).
Organelles
are required to be unequally distributed in certain situations and so motor
proteins and tracks are needed to set up and maintain this distribution against
chaotic influences. Secretary vesicles,
for example are required only at the synapse and so are taken there by axonal transport
(by kinesin). The golgi apparatus
performs an assembly line function where proteins are processed in a linear
fashion one modification taking place only after another is completed. This process is made more efficient by
having specific vesicles arranged on microtubules in the cis - trans
configuration with intermediate vesicles shuttling products between them.
Why have no Motor
Proteins associated with Intermediate Filaments been discovered?
In order for a motor protein to do useful work, some
directing influence must exist. In the case of the other two systems MT and MF,
the motors are directed my the polarity of the polymers, however IFs are not
polar and so it is difficult (but not impossible) to imagine how a motor could
proceed along an IF in one direction
Motor proteins evolved very early in the Eukaryotes and today many
protozoans express a great variety of MT and MF motor proteins, however, the IF
system is much more recent (probably) being absent from the protozoan and
possibly from the arthropods.
Consequently, it may be that IFs have not been around long enough for
specific IF motor proteins to evolve, also this function would seem to be
adequately fulfilled by MTs and MFs.
References:-
Microtubule Motors Barton, N.R. & Goldstein, L.S.B. “Going mobile:
Microtubule motors and chromosome
segregation.” Proc.Nat.Acad.Sci. USA.
March 1996. 93,
1735-1742.
Goodson
HV, Valetti, C & Kreis, T.E. Current Opinion in Cell Biol. Feb. 1997. 9,
18-28.
MT & MF motors Allan, V. “Membrane traffic motors” FEBS letters 369, 101 1995.
Kinesin Scholey,
J.M. “Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles.”
Journal of Cell Biology April 1996.
133, 1-4.
Moore,
J.D. & Endow, S.A “Kinesin proteins: a phylum of motors for microtubule-
based
motility” BioEssays 18 207,
April 1996.
Brady,
S.T. “A kinesin medley: biochemical and functional heterogeneity” Trends in Cell Biology
5, 159 April 1995.
Please direct any questions to me at:-
Room 444 or lab 446 fourth floor HRB. Tel (0131) 650 3714 or 3712. E-mail SKM@srv4.med.ed.ac.uk