An Introduction to the Cytoskeleton.
CS1
The cytoskeleton is a three dimensional network of filamentous protein which fills the space between organelles and gives shape and structure to cells. The cytoskeleton also provides the cell with “motility”, that being the ability of the entire cell to move and for material to be moved within the cell.. Three main protein systems constitute the cytoskeleton, these are (in order of typical abundance):- Microfilaments, Intermediate filaments and Microtubules. Although the term “cytoskeleton” is well used and accepted it unfortunately gives an impression of a rather static entity whereas all three constituents are dynamic structures, they constantly change shape through cycles of polymerisation / depolymerisation and interactions with other proteins.
Microfilaments
Microfilaments are linear assemblages of the 43 Kilodalton
protein actin. Actin is the most abundant protein in typical eukaryotic cells,
accounting for as much as 15% of total protein. It is a highly conserved
protein: the amino-acid sequence of actin from Acanthamoeba, a small soil amoeba, is 95% identical to vertebrate
isoforms of actin. X-ray crystallography has revealed that the actin monomer is
approximately pear shaped, and when viewed conventionally with the more pointed
end upper most, both the NH2 and the COOH termini are seen in the
bottom right hand corner.
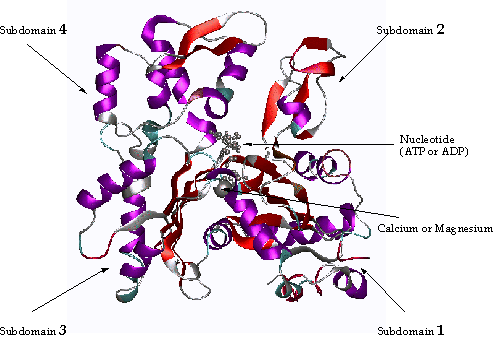
Figure 1 An Actin
Monomer ( courtesy of Bill)
Actin is composed of four domains with a large cleft almost
bisecting the molecule. This cleft
forms both a divalent cation (most likely magnesium in cells) and nucleotide
binding site. Because the actin subunit has polarity, the microfilament also
has (figure 2). Traditionally, the ends
of the microfilament have been referred to as "pointed" and
"barbed". This nomenclature
arises from the resemblance of microfilaments decorated with fragments of
myosin II to arrowheads in the electron microscope. Happily, this nomenclature coincides with the pointed appearance
of the actin monomer! (see figure 1 above, top is pointed end). The microfilament is a single-stranded helix
with each monomer rotated 166o with respect to neighbouring subunits
which means that every 36 nm, or every 13 subunits, subunits eclipse each other
at what appears to be a crossover.

Figure 2 A Microfilament
Microtubules
Microtubules (MTs) are assemblages of 110-kDa tubulin
dimers. Each dimer is actually a
heterodimer, i.e. the polymerising subunit is one 55-kDa a-tubulin
associated with one 55-kDa b-tubulin. As their
name suggests MTs are small tubes. They
are 25nm in diameter with an internal diameter of 14nm. It is not known if materials are transported
within the lumen of the MT, (this is unlikely as the ends at the cell centre
are most likely blocked) so MT perform a scaffold function rather that that of
a pipe. Note that the MTs are polar,
i.e. they have a b-tubulin exposed at the minus end and an a-tubulin
exposed at the plus end. Each MT is
typically composed of 13 tubulins arranged around the circumference, but some
MTs (especially those found in protozoans) exist which break this general rule.
Figure 3
Intermediate Filaments
Intermediate Filaments (IFs), are so called because, at 10nm in diameter they are typically intermediate in size between microfilaments and microtubules. IFs are different to microfilaments and microtubules in a number of fundamental respects. First of all they tend to be more or less permanent structures in tissues such as skin and hair, in fact in these non-living tissues IF proteins are almost the only protein. Thus it is true (but a little sad) to say that beauty is only IF thick! In other cell types, IFs are modified by phosphorylation when they are required to be disassembled for example during cell division. Unlike the highly conserved actins and tubulins more than 40 distinct IF proteins are encoded by a number of genes in mammalian cells. All IF proteins have a similar structure with a central helical rod domain and more variable head and tail domains. The IFs can be divided into five major classes:-
Class Name Tissue
i Acidic Keratins Epithelia
ii Basic Keratins Epithelia
iii Desmin Muscle
Glial Glial
cells and astrocytes
Peripherin Peripheral
neurones
Vimentin Mesenchyme
iv Neurofilaments Neurons
v Lamins Nuclear envelopes

Figure 4. A representation of the domain structure of the intermediate
filament family. The numbers refer to
the number of amino-acids typically forming each domain. The tail region is the most diverse, some
IFs do not have any, while others (neurofilament-H), has a tail of 607
amino-acids. IF monomers assemble in a
parallel fashion:-
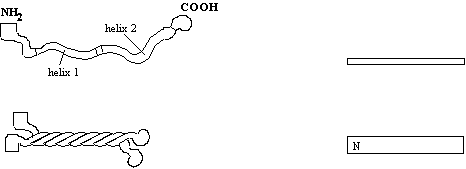
Dimerisation takes place by coiled-coil interaction of the a-helical
domain. The two helices (top left)
associate with those of another molecule wrapping around each other (bottom
left), so that the N terminus and C terminus lie next to each other. The rectangles to the right give a simplified
view for later comparison with higher order structures.
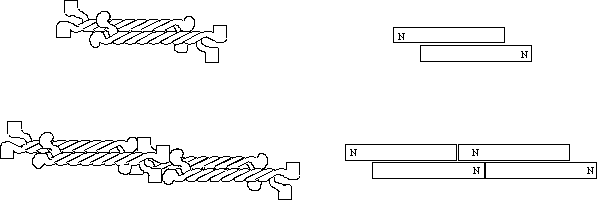
The IF dimers now associate with other dimers in an anti-parallel fashion so that there are now two N and two C termini at each end of the complex to form a tetramer (top). The next step is the association of the N terminal head of one tetramer with the C terminal tails of another. IF assembly can then proceed in this manner infinitely. It should be noted that the above scheme is somewhat tentative and lacks firm evidence. It is not clear for example exactly which domains are responsible for tetramers binding end to end.
Figure 5 Intermediate filament structure and
assembly
Cellular Organisation of the Cytoskeleton
The three cytoskeletal components have distinct sub-cellular
localisations. Microfilaments are
enriched in a layer known as the “cell cortex”, immediately beneath the plasma
membrane, and in cell projections such as microvilli. Microtubules extend from the perinucleus towards the cell
periphery. The plus ends of MTs point
to the cell periphery. IFs are
distributed in a similar pattern to MTs except where cells are in contact where
the IFs are enriched. IFs and MTs are
excluded from the actively expanding leading edge of the moving or “ruffling”
cell. In some situations a
co-localisation of the cytoskeletal systems is seen. For example, neurofilaments and MT co-localise in the axon of
neurons where specific cross links are made between the two systems. Cross linking proteins such as “filamin”
also exist which bind both MTs and MFs.
MFs are also associated with a number of other structures in specific
situations, such as the contractile ring in dividing cells, and in specific
cell types such as the “focal contact” in fibroblasts,
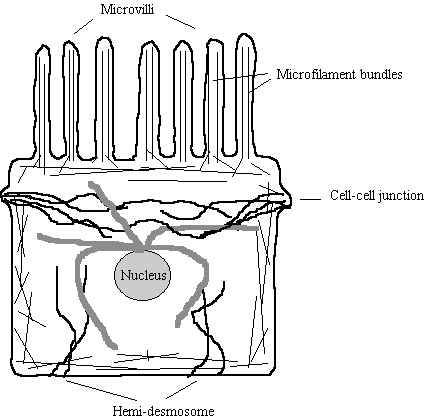
Figure 6. Distribution of Cytoskeletal
Systems in a typical Cell
Thin
straight lines - Microfilaments. Arranged in bundles in microvilli
Wavy
thicker black lines -
Intermediate Filaments. Connect cell to cell
Wavy
thick grey lines -
Microtubules. Radiate from perinuclear
MTOC.
Summary
Microfilaments and Microtubules are similar in many
respects, they are polar, dynamic structures whose assembly state is nucleotide
dependent and both interact with a host of associated proteins . IFs are apolar and rather more static
polymers which are depolymerised by phosphorylation. The three systems differ in their mechanical properties; MFs form
visco-elastic gels; MTs resist bending and compression; IFs are extremely tough
fibres which resist stretching. MFs are arranged as gels or bundles in
association with a large number of actin binding proteins. MTs are usually single typically have their
minus end associated with an MTOC deep within the cell, with the plus end
toward the periphery. IFs connect
cell-cell junctions to give strength to tissues. All three systems are interconnected to various extents.
References:-
General Molecular
Biology of the Cell chapter 16, p787. (for third edition)
Bray,
D. Cell Movements Garlands press
(1992).
Intermediate filaments Stewart, M. Current Opinion Cell Biol. 5, 3-11 (1993).
Please direct any questions to me at:-
Room 444 or lab 446 fourth floor Hugh Robson
Building. George Square.
Tel (0131) 650 3714 or 3712. E-mail SKM@srv4.med.ed.ac.uk