Cytoskeletal Dynamics. CS2
The three cytoskeletal components differ in the way that
they polymerise. Microtubules and
microfilaments share many properties of assembly, but intermediate filaments
are fundamentally different. MTs and
MFs bind GTP and ATP respectively which hydrolyses when these proteins
polymerise. However, many other
proteins including IFs and bacterial flagellin (which produces the flagella),
polymerise without a requirement for nucleotide hydrolysis. The nucleotide hydrolysis is the price the
cell must pay in order to allow rapid remodelling of these cytoskeletal
elements. For technical (and historic)
reasons most in vitro information is
available from the microfilament systems, whereas the microtubule field is well
ahead in in vivo studies. Comparatively little is known about IF
dynamics. This discussion will
therefore concentrate on the actin and tubulin systems.
Actin Polymerises to produce Microfilaments
In physiological conditions, actin monomers (G-actin)
spontaneously self associate to form microfilaments (F-actin). This polymerised
form of actin is in constant equilibrium with G-actin. Monomers are able to add
to the ends of filaments, form nuclei with another two monomers to create new
filaments, and to leave filaments. The polarity of actin subunits within
microfilaments means that the two ends are topologically different, the narrow
face of actin subunits is exposed at the pointed end while the more bulbous
face containing the NH2 and COOH termini is exposed at the barbed
end. Pure actin can be switched between these states in the test-tube by
altering the salt concentration. Actin
in low salt conditions is in the G-state, while adding salts causes the actin
to polymerise.

Figure 6
Polymerisation is a multi-step process. The first two steps are highly unfavourable. Only the trimer is more likely to lead to the next step to the right than to disassemble. Because the early stages are improbable the time course of polymerisation is multi-phasic, starting off slowly accelerating and finally, as the amount of free G-actin becomes limiting, the rate decreases.
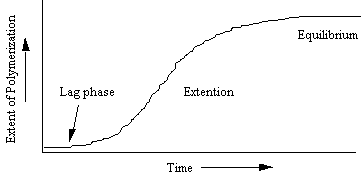
Figure 7. After addition of salts at time zero, there is a
lag phase during which the rate limiting nucleus for polymerisation form. These nuclei consist of three actin monomers
associating to form the smallest polymerising unit. This step is highly unfavourable, but after these have formed,
polymerisation by extension is favoured and rapid. The rate of extension gradually lessens as the available monomers
become exhausted. An equilibrium is
then established when the number of actin monomers joining the filaments equals
that of monomers leaving the filaments
Tubulin
Polymerises to produce Microtubules
The
assembly of MTs is less clear but is thought to involve a similar process to
that of actin (above). Tubulin dimers
are stable, tightly bound structures under normal conditions and are the
polymerising subunit. The intermediate
steps are not known, but the formation of a tubulin nucleus is much less
favourable than is the case for actin and so the lag phase is longer. In fact the nucleation step is so unlikely
that MTs in cells grow from specialised structures known as MicroTubule Organising
Centres (MTOC).
Actin and Tubulin
Hydrolyse Nucleotide during Polymerisation
In both cases hydrolysis takes place after the particular
subunit has joined the polymer but in neither case is nucleotide hydrolysis
required for polymerisation. Actin
which has ADP bound polymerises albeit more slowly that is the case for ATP and
polymerisation can also take place when actin has a non-hydrolysable analogue
of ATP in the binding site. The same is
true for GDP-bound tubulin. The energy
dissipated in the hydrolysis is used to destabilise the polymers. In both cases the Di-phosphate nucleotide
containing polymer is much less stable than the Tri-phosphate state and so is
likely to depolymerise. Therefore
nucleotide hydrolysis allows polymer formation at the same time and place as
polymer disassembly. This is crucially
important for a wide variety of cell movement which will be discussed in
following lectures.
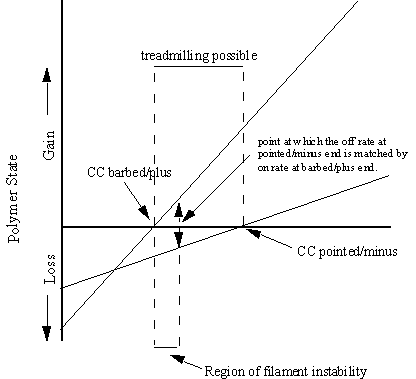
Figure 8. The polymer state as a function of the concentration of
protein present. In polymerising conditions
no polymer is observed until the monomer concentration (ordinate) exceeds that
of the critical concentration (CC) of the barbed/plus end of the MF or MT. However the filament formed would not be
stable until after the point indicated above, where the off rate at the
pointed/minus end equals that of the barbed/plus end. The model is equally true for both MTs and MFs, however
differences between the two systems mean that some properties are difficult to
see in one system while being very apparent in the other. Treadmilling is discussed later.
Dynamic
Instability
This is a phenomenon known to occur in microtubules which
has been observed in vivo as well as in vitro. Although it is theoretically possible that this also occurs in
microfilaments it should become clear that the differences between the systems
make this less likely and/or less important than is the case for MTs. Individual MTs can be seen in very thin
processes in cells, and in most cells using fluorescence microscopy because of
their relative large diameter and due to their general lack of abundance. It is only possible to see individual MFs
with fluorescence microscopy using very dilute preparations. In cells some MTs are seen to grow, yet
other MTs close by are seen to dramatically shorten and sometimes to
disappear. In other cases a rapidly
shortening MT may suddenly start to grow.
This is “dynamic instability” and is explained (surprisingly easily perhaps)
by the state of bound nucleotide. It is
known that MTs depolymerise very rapidly if they are composed of GDP- bound a-b
dimers while being much more stable if composed of GTP dimers. As dimers (usually GTP-dimers) may add to
either end of an MT this means that both ends are likely to have GTP dimers. If however the rate of GTP hydrolysis
exceeds the rate of dimer addition even transiently, GDP-dimers would be
exposed and the filament would rapidly disassemble.
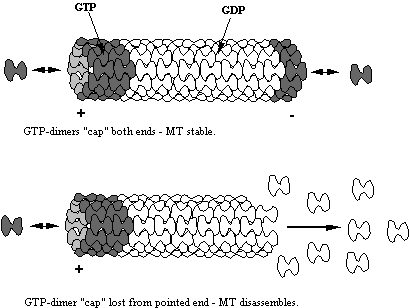
Figure 9
Treadmilling.
The barbed end of microfilaments is preferred for monomer
addition over the pointed end, where net disassembly is favoured. Within the region labelled “treadmilling
possible” in figure 8 page 6, a net flux of monomers occurs along the length of
the filament. Monomers add to the barbed end, travel the length of the filament
as other monomers add behind, and leave from the pointed end, this is called
this "treadmilling". There is
evidence that treadmilling may indeed occur close to the leading edge of motile
lamellipodium of fibroblasts, neuronal growth cones and this is probably true
for all expanding lamellae.
Interestingly, a microfilament in the process of treadmilling may
actually do work, this will be discussed later.

Figure 10. If the overall
filament size remains constant, addition at the barbed end and loss at the
pointed end means that any particular monomer is conveyed from barbed to
pointed end. There is good evidence
that such a mechanism actually takes place in cells and is responsible for the
protrusion of the leading edge of moving cells as well a type of vesicular transport
in cells, called "rocketing".
Microfilaments are
controlled by Actin Binding Proteins (ABPs)
Actin is bound by about 48 different types of actin binding
proteins. Many of these are present in
cells as a range of iso-types with subtly different actin binding properties
which makes the situation very complex indeed.
Some of the major types are diagrammed below:-
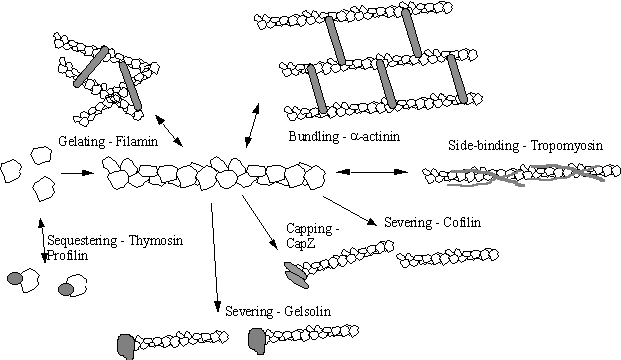
Figure 11 The
Actin Binding Proteins
Some ABP cannot simply be classified as belonging to just
one of the above broad classes, because they have properties found in more than
one category. Villin for example, a
member of the gelsolin severing proteins could be described as a severing
protein, a capping protein and even a bundling protein since all three
properties are displayed under the influence of a variety of second
messengers. Indeed a common theme in
the ABPs is that their actin binding is modulated or switched by second
messengers. Many are calcium sensitive
(gelsolin, a-actinin), some (capZ, filamin, gelsolin, profilin) are inhibited
by polyphosphoinositides such as PIP2, and a few (cofilin, EF1a,) are
pH dependent.
Microtubules are
controlled by Microtubule Associated Proteins (MAPs)
Less is known about these proteins than the ABPs, but many
of the known MAPs perform the same type of function as ABPs. Interestingly, some ABPs are also MAPs. EF1a for example bundles
microfilaments in a unique parallel, squared configuration and the same protein
severs microtubules, this is all in addition to its more traditional function
as an elongation factor in translation of mRNA! Four main types of MAPs have so far been characterised. Those which bind MTs and stabilise them
(such as MAP2 and Tau), those which nucleate assembly of MTs, MT severing
proteins and those which are MT dependent motor proteins. The motor proteins will be discussed
later. MT nucleating proteins include
another member of the tubulin family - g-tubulin, which is associated with
MTOCs.
References:-
General Molecular
Biology of the Cell chapter 16, p787. (for third edition)
Microtubules and MAPs McNally, F.J. (1996). Current Opinion in Cell
Biology. March 8, 23-29.
MF and MT dynamics Mitchison, T.J. (1992). Molecular
Biology of the Cell. 3,
1309-1315.
Please direct any questions to me at:-
Room 444 or lab 446 fourth floor HRB. Tel (0131) 650 3714 or 3712. E-mail SKM@srv4.med.ed.ac.uk