An Introduction
to the Cytoskeleton.
The cytoskeleton
is a three dimensional network of filamentous protein which fills the space
between organelles and gives shape and structure to cells. The cytoskeleton also provides the cell with
“motility”, that being the ability of the entire cell to move and for material
to be moved within the cell.. Three
main protein systems constitute the cytoskeleton, these are (in order of typical
abundance):- Microfilaments, Intermediate
filaments and Microtubules. Although
the term “cytoskeleton” is well used and accepted it unfortunately gives an
impression of a rather static entity whereas all three constituents are dynamic
structures, they constantly change shape through cycles of polymerisation /
depolymerisation and interactions with other proteins.
Microfilaments
Microfilaments
are linear assemblages of the 43 Kilodalton protein actin. Actin is the most
abundant protein in typical eukaryotic cells, accounting for as much as 15% of
total protein. It is a highly conserved protein: the amino-acid sequence of
actin from Acanthamoeba, a small soil
amoeba, is 95% identical to vertebrate isoforms of actin. X-ray crystallography
has revealed that the actin monomer is approximately pear shaped, and when
viewed conventionally with the more pointed end upper most, both the NH2
and the COOH termini are seen in the bottom right hand corner.
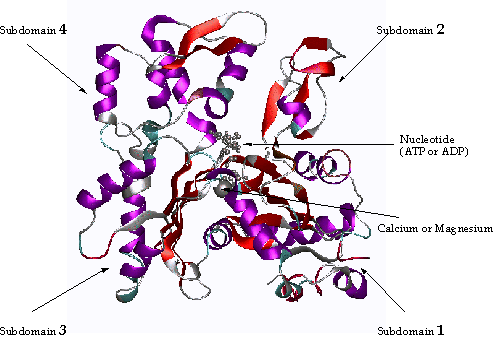
Figure 1 An Actin monomer
Actin is composed
of four domains with a large cleft almost bisecting the molecule. This cleft
forms both a divalent cation (most likely magnesium in cells) and nucleotide
binding site. Because the actin subunit has polarity, the microfilament also
has (figure 2). Traditionally, the ends of the microfilament have been referred
to as "pointed" and "barbed". This nomenclature arises from
the resemblance of microfilaments decorated with fragments of myosin II (see
later) to arrowheads in the electron microscope. Happily, this nomenclature
coincides with the pointed appearance of the actin monomer! (see figure 1
above, top is pointed end). The microfilament is a single-stranded helix with
each monomer rotated 166o with respect to neighbouring subunits
which means that every 36 nm, or every 13 subunits, subunits eclipse each other
at what appears to be a crossover.

Figure
2 A Microfilament
Microtubules
Microtubules
(MTs) are assemblages of 110-kDa tubulin dimers. Each dimer is actually a heterodimer, i.e. the polymerising
subunit is one 55-kDa a-tubulin associated with one 55-kDa b-tubulin. As their name suggests MTs are small
tubes. They are 25nm in diameter with
an internal diameter of 14nm. It is not
known if materials are transported within the lumen of the MT, (this is
unlikely as the ends at the cell centre are most likely blocked) so MT perform
a scaffold function rather that that of a pipe. Note that the MTs are polar, i.e. they have a b-tubulin exposed
at the minus end and an a-tubulin exposed at the plus end. Each MT is typically composed of 13 tubulins
arranged around the circumference, but some MTs (especially those found in
protozoans) exist which break this general rule.
Figure 3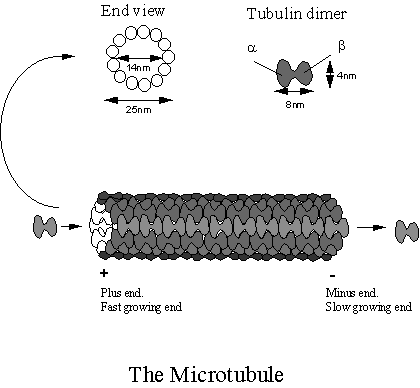
Intermediate Filaments
Intermediate
Filaments (IFs), are so called because, at 10nm in diameter they are typically
intermediate in size between microfilaments and microtubules. IFs are different to microfilaments and
microtubules in a number of fundamental respects. First of all they tend to be more or less permanent structures in
tissues such as skin and hair, in fact in these non-living tissues IF proteins
are almost the only protein. Thus it is
true (but a little sad) to say that beauty is only IF thick! In other cell types, IFs are modified by
phosphorylation when they are required to be disassembled for example during
cell division. Unlike the highly
conserved actins and tubulins more than 40 distinct IF proteins are encoded by
a number of genes in mammalian cells.
All IF proteins have a similar structure with a central helical rod
domain and more variable head and tail domains. The IFs can be divided into five major classes:-
Class Name Tissue
i Acidic
Keratins Epithelia
ii Basic
Keratins Epithelia
iii Desmin Muscle
Glial Glial cells and
astrocytes
Peripherin Peripheral neurones
Vimentin Mesenchyme
iv Neurofilaments Neurons
v Lamins Nuclear envelopes

Figure 4.
A representation of the domain structure of the intermediate filament
family. The numbers refer to the number
of amino-acids typically forming each domain.
The tail region is the most diverse, some IFs do not have any, while
others (neurofilament-H), has a tail of 607 amino-acids. IF monomers assemble in a parallel fashion:-
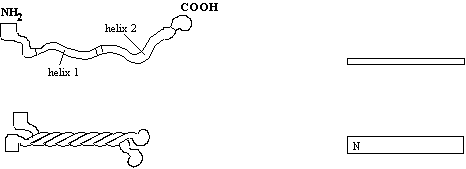
Dimerisation
takes place by coiled-coil interaction of the a-helical
domain. The two helices (top left) associate
with those of another molecule wrapping around each other (bottom left), so
that the N terminus and C terminus lie next to each other. The rectangles to the right give a
simplified view for later comparison with higher order structures.
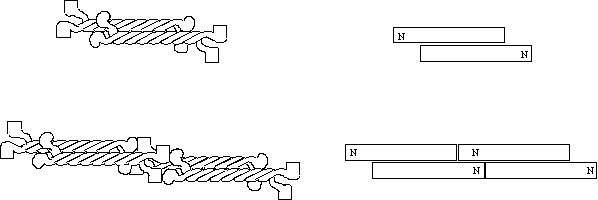
The IF dimers now associate with other dimers in an anti-parallel fashion so that there are now two N and two C termini at each end of the complex to form a tetramer (top). The next step is the association of the N terminal head of one tetramer with the C terminal tails of another. IF assembly can then proceed in this manner infinitely. It should be noted that the above scheme is somewhat tentative and lacks firm evidence. It is not clear for example exactly which domains are responsible for tetramers binding end to end.
Figure 5 Intermediate
filament structure and assembly
Cellular Organisation of the Cytoskeleton
The three
cytoskeletal components have distinct sub-cellular localisations. Microfilaments are enriched in a layer known
as the “cell cortex”, immediately beneath the plasma membrane, and in cell
projections such as microvilli.
Microtubules extend from the perinucleus towards the cell periphery. The plus ends of MTs point to the cell
periphery. IFs are distributed in a
similar pattern to MTs except where cells are in contact where the IFs are
enriched. IFs and MTs are excluded from
the actively expanding leading edge of the moving or “ruffling” cell. In some situations a co-localisation of the
cytoskeletal systems is seen. For
example, neurofilaments and MT co-localise in the axon of neurons where
specific cross links are made between the two systems. Cross linking proteins such as “filamin”
also exist which bind both MTs and MFs.
MFs are also associated with a number of other structures in specific
situations, such as the contractile ring in dividing cells, and in specific
cell types such as the “focal contact” in fibroblasts,
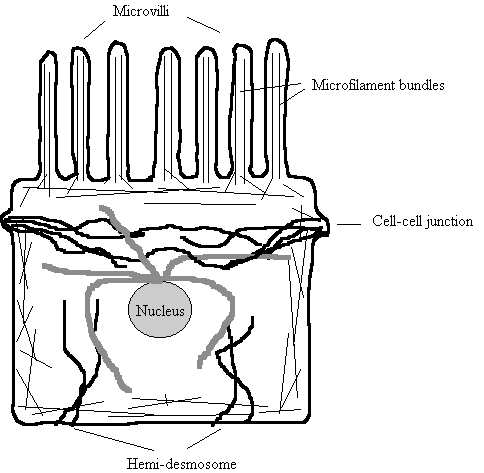
Figure 6. Distribution
of Cytoskeletal Systems in a typical Cell
Thin straight lines - Microfilaments. Arranged in bundles in microvilli
Wavy thicker black lines - Intermediate Filaments.
Connect cell to cell
Wavy thick grey lines - Microtubules. Radiate from perinuclear MTOC.
Summary
Microfilaments
and Microtubules are similar in many respects, they are polar, dynamic
structures whose assembly state is nucleotide dependent and both interact with
a host of associated proteins . IFs are
apolar and rather more static polymers which are depolymerised by
phosphorylation. The three systems
differ in their mechanical properties; MFs form visco-elastic gels; MTs resist
bending and compression; IFs are extremely tough fibres that resist stretching.
MFs are arranged as gels or bundles in association with a large number of actin
binding proteins. MTs are usually
single typically have their minus end associated with an MTOC deep within the
cell, with the plus end toward the periphery.
IFs connect cell-cell junctions to give strength to tissues. All three systems are interconnected to
various extents.
References:-
General Molecular
Biology of the Cell chapter 16, p787. (for third edition)
Bray, D. Cell Movements Garlands press (1992).
Intermediate filaments Stewart, M. Current
Opinion Cell Biol. 5, 3-11 (1993).
The Cytoskeleton & Movement of Vesicles/Organelles.
It is often
necessary for vesicles and organelles to be transported to other parts of the
cell. An obvious example of this is the
axon of a nerve cell which may be yards long.
Vesicles full of neurotransmitters, synthesised in the cell body must
travel down the length of the axon to be delivered at the synapse. Most metabolites in cells merely diffuse
throughout the cell and don’t need specific conduits, but organelles and
vesicles are too large for diffusion to be useful since they would become
entangled in the cytoplasm and other organelles. Also, a simple diffusive mechanism would oblige an equal distribution
of all organelles and vesicles to exist in all cell types; an obviously
intolerable situation.

Diffusion cannot account for vesicle transport (see Bloom & Goldstein, 1998)
The equation for
three dimensional diffusion is:- and
for one dimensions:-
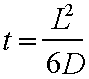
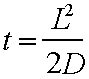
Where t is the time taken to travel the
length L and D is the diffusion constant
2.5x10-10 cm2/sec for a 200nm diameter neutrophil
granule ( Felder & Kam, 1994). The diffusion constant was arrived at by
measurement in neutrophils devoid of microfilaments or microtubules. In an average cell this means that it would
take about 10 minutes to diffuse from the Golgi to the PM in the absence of a cytoskeletal networks that
would otherwise impede it. This one
imagines may be sufficient but if one does the same calculation for a 1m long
axon this figure (1 D diffusion) some 630,000 years would be required to
transport a vesicle from one end to the other (this is slower that the west
coast Virgin service through Carlisle!)
There are two
mechanisms by which the cell might produce localised distribution of vesicles,
one is to allow the vesicles unhindered access to all parts of the cytosol, but
to trap them when they arrived at that particular region, alternatively the
cell may move the vesicles to specific regions. Cells seems to adopt both mechanisms although the difference
between the two methods is not always apparent.
Vesicular and organelle motility is
most obviously associated with the microtubule system (see second lecture
handout MPT-10). Microtubules are ideal candidates for the
provision of tracks along which cargoes may be transported since they are
rigid, long, straight and polar. Actin
is not straight (unless it is bundled), and often arranged in orthogonal gels
(not polar). However microfilaments do
provide tracks and anchors for organelles in a number of important situations.
Microtubules
are ideally suited as tracks to deliver vesicles and organelles to distant
cellular regions. They are rigid and so
comparatively straight, they are polar with the plus end almost invariably
pointing to the periphery. Two main
groups propel vesicles and organelles along MT in the cytoplasm; cytoplasmic
dynein and a more diverse group, the kinesins
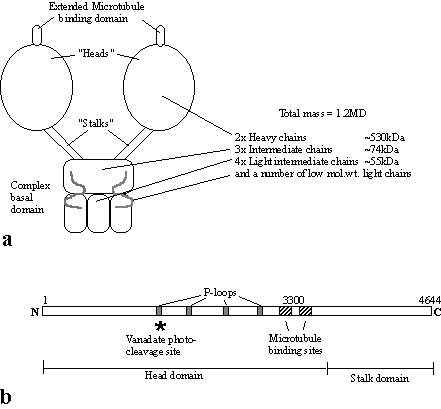
Cytoplasmic Dynein
Cytoplasmic
dynein is also known as MAP1C, it is a huge, two headed ATP-ase which is minus
end directed. Some dyneins from the
flagella of Chlamydomonas and Tetrahymena have three heads, but most
other flagellar dyneins have just the two.
Cytoplasmic dynein moves vesicles at around 0.3 µm/sec in vitro, is unaffected by NEM and
Vanadate and can utilise ATP, GTP or ITP for force production. The enormous size of the dynein molecule has
meant that our understanding of its structure has not progressed as fast as
kinesin. The heavy chain is 4644 amino
acids in rat and smaller in both Dictyostelium
and Saccharomyces, about 3300 AA of
this form the “head” while the remainder form the stalk (compared to 340 AAs for
the kinesin head and 850 AAs for myosin).
|
|
The dynein heavy
chain contains 4 conserved nucleotide-binding “P-loop” motifs. Only the first of these P-loops is actually
involved in nucleotide hydrolysis, but the others may also bind nucleotide,
perhaps to regulate the molecule (Mocz & Gibbons, 1996). It is known that vanadate is capable of
cleaving the dynein (and other ATPases) at the ATP binding site, this is, as
expected in the first P-loop. The
microtubule binding site is exposed where one might imagine it would be on the
three dimensional structure of the dynein molecule, at the top, on an extended
structure (see part a) (Gee et al,
1997). However, the microtubule site is
encoded by a stretch of sequence toward the C-terminus, next to the stalk domain. Solving the 3D structure of the dynein
molecule will be a major task! Not much
is presently known about the regulation of dynein at the molecular level. What is known is largely phenomenological,
for example it is known that okadaic acid (an inhibitor of phosphatases PP1 and
PP2A) causes a 27 fold increase in the number of ER tubules moving on
microtubules in an in vitro
reconstituted experiment (Allan, 1995), but it is not known which of the many
proteins in the complex that gets phosphorylated is the regulating
component.
Figure 7
The Kinesins
The kinesins are
a large group of microtubule based motor proteins that are formed from
multi-component complexes. The first
kinesin was identified in the axon in 1985 but several other kinesin related
proteins (KRP) have come to light.
Three kinesin subfamilies have been implicated in the plus end
directed movement of vesicles and organelles. All three share a similar kinesin “head” domain but are otherwise
quite different. The kinesin head
domain has a fold with surprising similarity to the myosin head and some switch
type G-proteins (Vale, 1996). Although
the kinesins carry vesicles it is suspected that each type carries a specific
sub-set of vesicle. Kinesin moves
vesicles at around 1 µm/sec, is inhibited by NEM and Vanadate and can only
utilise ATP for force production
Many other kinesin related motor
proteins have been discovered which are involved in the spindle pole formation
and karyokinesis. These have largely
been identified at the gene level by genetic screen and so not much is known
about their biochemical properties.
However, one of these, NCD is a kinesin related protein which drives
minus end movement (see below).
______________________________________________________________________________________________________
Protein direction Mol.Wt (kDa) Speed Cargo
of heavy chain (µm/min)
______________________________________________________________________________________________________
Kinesin plus 110 (heterotetrameric) 30-54 Vesicles
Unc-104 (KIF1A) plus 192 (monomeric) 72 precursor vesicles
(KIF1B) plus 130
(monomeric) 40 Mitochondria
Kinesin II (KRP85/95) plus 79 (heterotrimer) 24 Vesicles
Rabkinesin-6 ? ~100 (?) ? Golgi & TGN
ncd minus ~100 chromosomes
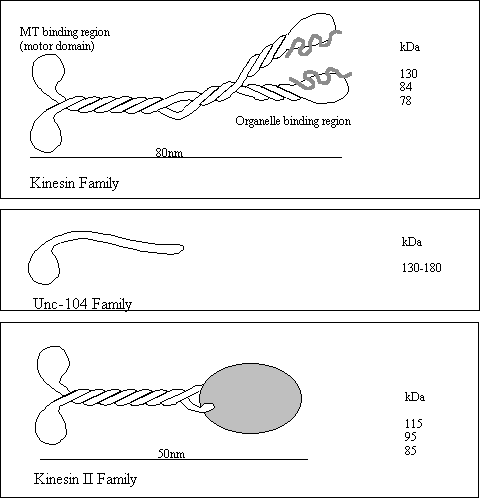
Figure 8
Control of Dynein and Kinesin motor activity.
In most cell
types, MTs have their plus ends facing the plasma membrane and the minus end
associated with a MTOC deep in side the cell.
Kinesins will therefore generally move organelles toward the
plasma-membrane and dyneins take them towards the nucleus. A spectacular example of the is the movement
of pigment granules along the MT in the chromatophore from the skin of a variety of fish and
amphibians. Chromophores are stellate
flattened cell types which contain a multitude of dense light absorbing
granules. When the granules are packed
in the centre of the cell, the skin appears light, but darkens when the pigment
granules are dispersed throughout the cell.
This affords the animal protective camouflage. Granule movement requires microtubules. Dispersion depends on cAMP or IP3/diacylglycerol, the activity of
PKA or PKC, and kinesin. Aggregation
depends on phosphatase activity and it thought to be dynein dependent, although
this step is very rapid at 5µm/sec and dynein driven motility is only 0.3
µm/sec in vitro. Cellular cofactors absent from the in vitro studies may account for this
difference.
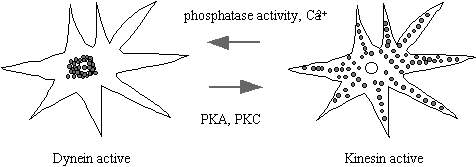
Figure 9
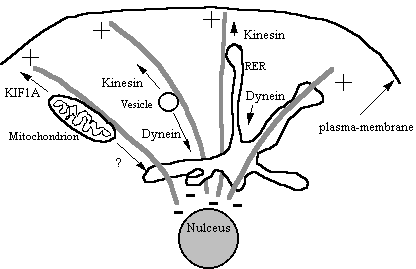
Figure 10
Other membranous
organelles such as the Gogli, RER, lysosomes and mitochondria are moved around
the cell on microtubules. Many of these
share the same motors so it is difficult to see how the cell switches on
motility of one particular organelle and not others. Perhaps motor receptors are the key (see later), localised
phosphorylation of motor protein complexes is perhaps another controlling
mechanism.
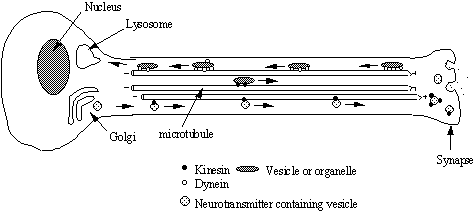
Figure 11
Transport of
material along the nerve axon.
Materials such as neurotransmitter peptides are synthesised in the cell
body and sequestered in vesicles at the golgi.
These vesicles are then transported down the axon towards the synapse by
kinesin motor proteins. This distance
may be yards in the case of a giraffe sciatic nerve! Other materials are transported from the synapse to the cell body
by dynein motors (see Nixon 1998 for a recent review of transport of
cytoskeletal components)
.
|
Figure 12 |
Are there specific Kinesin and Dynein receptors on cargo
membranes?
Recently a
putative receptor “kinectin” has been identified by passing
detergent-solubilized microsomal membranes over a kinesin affinity column (see
Burlhardt, 1996). Kinectin has a
molecular weight of 160 kDa and the cloned cDNA reveals that the protein has a
hydrophobic N-terminus and a high probability of forming coiled-coil Kinectin has numerous putative
phosphorylation sites and is known to be phosphorylated on serine, however
there has been no correlation between the state of phosphorylation and kinesin
binding activity to date. Kinectin is
most abundant on the ER membranes, and is not thought to bind dynein. It is very likely that receptors other than
kinectin exist in or on other membranes.
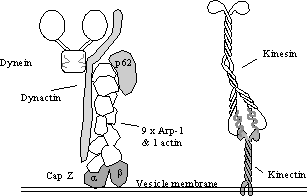
A possible candidate for a dynein receptor is “dynactin”, a 150-170 kDa
protein complex (see left). At least
two mRNAs and therefore two proteins are produced from the kinectin gene in
humans and this may allow a measure of specificity. Early evidence suggests that kinectin is present of E.R. but not
Golgi complex membranes.
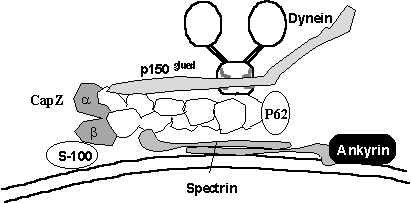
The Golgi complex membrane skeleton and motor proteins.
It has very
recently come to light that the Golgi complex has its own, Golgi-specific
isoforms of a number of proteins involved with the actin (and microtubule to a
lesser extent) cytoskeleton:-
____________________________________________________________
Protein Function
____________________________________________________________
Spectrin a linker between other
membranous proteins.
Ankyrin Binds 4.1 type proteins
and spectrin
ARP1 a component of the dynactin
complex
Comitin an “annexin” actin and
phospholipid binding protein
|
Figure 13 |
Other
Golgi-specific protein isoforms are also expected to exist (Beck & Nelson,
1998), permitting the postulation of a scheme whereby dynein may be targeted to
Golgi membranes (see left). The
dynactin complex is composed of a short “filament” of 9 ARP1 molecules. ARP1 is
an actin related protein that is known to bind Golgi spectrin and so it is
tempting to speculate that this may form the basis for a dynein receptor. Additionally, S-100 proteins bind CapZ which
it turn binds the ARP1 mini-filament, CapZ also binds phosphatidyl- inositol
4,5-bisphosphate which may also be relevant.
The binding partner for golgi specific ankyrin has not yet been
identified, but there are many splice variants of Band4.1 proteins known to
bind ankyrin in other membranes and there are many Band4.1 related proteins.
Regulation and specificity mediated by G-proteins
Many events such
as fusion, and recognition of membranes is brought about by an enormous family
of G-proteins, particularly the G proteins of the rab family (see Martinez
& Goud 1998). Rab6 is just one of
these numerous proteins, it is restricted to Golgi and the TGN, recently, a
kinesin related protein which binds to Rab6 has been identified (Rabkinesin-6;
Echard et al, 1998) so it seems possible that the plus end directed
kinesin-like activity already detected in the golgi, is localised and
controlled by Rab6. As Rabkinesin-6 has
very limited homology to the other kinesins, it is possible that there are many
of these in the genome that are also too little conserved to be recognised in
EST libraries and other sequencing databases.
Why are organelles arranged and moved along MTs and MFs?
Organelles are
required to be unequally distributed in certain situations and so motor
proteins and tracks are needed to set up and maintain this distribution against
chaotic influences. Secretary vesicles,
for example are required only at the synapse and so are taken there by axonal
transport (by kinesin). The golgi
apparatus performs an assembly line function where proteins are processed in a
linear fashion one modification taking place only after another is completed. This process is made more efficient by
having specific vesicles arranged on microtubules in the cis - trans
configuration with intermediate vesicles shuttling products between them.
Co-operation between Microtubules and Microfilament systems
in vesicular transport
In many cases
there appears to be considerable overlap in the vesicular motility driven by
the MT and MF systems. Mitochondria are
moved along MTs by KIF1B, and along MFs at 1.4µm/min by a myosin I-like
activity (at least in yeast, see bottom of page 7 on previous handout). Also yeast cell with disabled kinesin are
rescued by overexpression of myosin I!
The sea urchin coelomocyte has been mentioned (page 6) in connection
with transport of vesicles towards the cell periphery upon stimulation. In addition to using microfilaments as
track, these same cells use microtubules to transport mitochondria, in fact the
motility of the mitochondria is increased 1.5 fold in the absence of actin
filaments suggesting that the presence of the filaments otherwise impedes there
transport possibly as a result of the increased cytosolic viscosity (Krendel et
al, 1998). The two motor protein tracks, microfilaments and microtubules
support seemingly exactly the same type of vesicular traffic in different cell
type. Melanin containing vesicles for
example, are transported by Myosin V along microfilaments in mammalian melanoma
cells while they are moved along microtubules in fish skin. Two very similar organisms, Reticulamyxa and Laberinthula also demonstrate this duplicity. Both organisms are large syncytical “amoeba”
living in fresh and salt water respectively.
Reticulamyxa moves its nuclei
and other organelles around on a vast arrays of microtubule bundles, while Laberinthula has bundles of actin to
perform the same function.
Why have no Motor Proteins associated with Intermediate
Filaments been discovered?
In order for a
motor protein to do useful work, some directing influence must exist. In the
case of the other two systems MT and MF, the motors are directed my the
polarity of the polymers, however IFs are not polar and so it is difficult (but
not impossible) to imagine how a motor could proceed along an IF in one
direction Motor proteins evolved very
early in the Eukaryotes and today many protozoans express a great variety of MT
and MF motor proteins, however, the IF system is much more recent (probably)
being absent from the protozoan and possibly from the arthropods. Consequently, it may be that IFs have not
been around long enough for specific IF motor proteins to evolve, also this
function would seem to be adequately fulfilled by MTs and MFs.
References:-
Allan, V. Membrane
traffic motors. FEBS letters 369,
101 1995.
Barton, N.R. &
Goldstein, L.S.B. Going mobile: Microtubule motors and chromosome segregation. Proc.Nat.Acad.Sci. USA. March 1996.
93, 1735-1742.
Brady, S.T. A
kinesin medley: biochemical and functional heterogeneity. Trends in Cell Biology 5, 159 April 1995.
Beck, K.A.,
Buchanan, J.A., and Nelson, W.J. (1997) Golgi membrane skeleton:
identifiaction, localization and oligomerization of a 195 kDa ankyrin isoform
associated with the golgi complex. J.Cell
Sci. 110; 1239-1249.
Beck, K.A., and
Nelson, W.J. (1998) A spectrin membrane skeleton of the golgi complex. Biochim.Biophys.Acta. 1404;
153-1160.
Burkhardt, J.K.
(1996) In search of membrane receptors for microtubule-based motors - Is
kinectin a kinesin receptor? Trend Cell
Biol. 6; 127-131.
Echard, A.,
Jollivet, F., Martinez, O., Lacapere, J.-J., Rousselet, A., Janoueix-Lerosey,
I., Goud, B. (1998) Interaction of a golgi-associsted kinesin-like protein with
Rab6. Science 279; 580-585.
Gee, M.A., Heuser,
J.E. and Vallee, R.B. (1997) An extended microtubule-binding structure within
the dynein motor domain. Nature 390;
636-639.
Goodson HV,
Valetti, C & Kreis, T.E. Current
Opinion in Cell Biol. Feb. 1997. 9, 18-28.
Hirokawa, N. (1998)
Kinesin and dynein superfamily proteins and the mechanism of organelle
transport. Science 279;
519-526.
Krendel, M.,
Sgourdas, G., Bonder, E.M. (1998) Disassembly of actin filaments leads to
increased rate and frequency of mitochonrial movement along microfilaments. Cell Mot.Cytoskeleton. 40;
368-378.
Mocz, G. and
Gibbons, I.R. (1996) Phase partition analysis of nucleotide binding to axonal
dynein. Biochemistry 35; 9204-9211.
Moore, J.D. & Endow,
S.A “Kinesin proteins: a phylum of motors for microtubule- based motility” BioEssays 18 207, April 1996.
Nixon, R.A. (1998)
Dynamic behaviour and organization of cytosketal proteins in neurons:
reconciling old and new findings BioEssays,
20; 798-807.
Scholey, J.M.
“Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles.” J.Cell Biol. 1996. 133, 1-4.
Stow, J.L. and
Heimann, K. (1998) Vesicle budding on Golgi membranes: regulation by G proteins
and myosin motors Biochim.Biophys.Acta.
1404; 161-171.
Weiner, O.H.,
Murphy, J., Griffiths, G., Schleicher, M. and Noegel, A.A. (1993). The
actin-binding protein comitin (p24) is a
component of the
Golgi apparatus. J.Cell Biol. 123; 23-34.