Myosins and
Disease
The myosin are a diverse group of motor proteins which
interact with microfilaments to produce force.
What defines a myosin is a conserved “head” domain which binds actin and
hydrolyses Mg-ATP. This head domain is
the motor. A generalised scheme of the actin-myosin cycle is outlined
below. There are at least 13 classes of
myosins but this number is likely to be very much greater (see figure 3). These have been identified by cDNA
technology (largely RT-PCR), and very scant biochemical details have been
gleaned from these different classes with the exception of myosin II and myosin
I. Fortunately these same classes
represent the most abundant forms and possibly the two most divergent.

Figure 1.
The Myosin
Cross-bridge Cycle. A. ATP binding to a cleft at the “back” of the
head causes a conformation which cannot bind actin. B. As the ATP is
hydrolysed, the head swings back about 5nm to the “cocked” position the ADP and
Pi remain bound. C+D. The force generating stages. When the Pi leaves the myosin, the head
binds the actin and the “power stroke” is released as the head bind actin. ADP is released to continue the cycle. At
this stage the head in bound to actin in the “rigor” or tightly bound state.
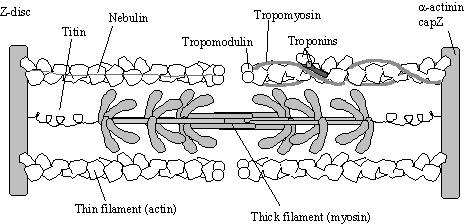
Figure 2.
The sarcomere
in a semi-contracted state. As myosin
is activated this arrangement causes the Z-discs to move towards each
other. The troponin complex senses
calcium and forces tropomyosin to move position on the filament which controls access
to the myosin heads. Myosin thick
filaments are arranged as anti-parallel bundles each with a central bare
zone. These thick filaments are
associated with “protein C” which presumably helps keep the structure
together. Thick filaments are held to
the Z-disc by Titin, a giant elastic protein.
Thin filaments are stabilised by nebulin, and held to the Z-disc by a-actinin and capZ.
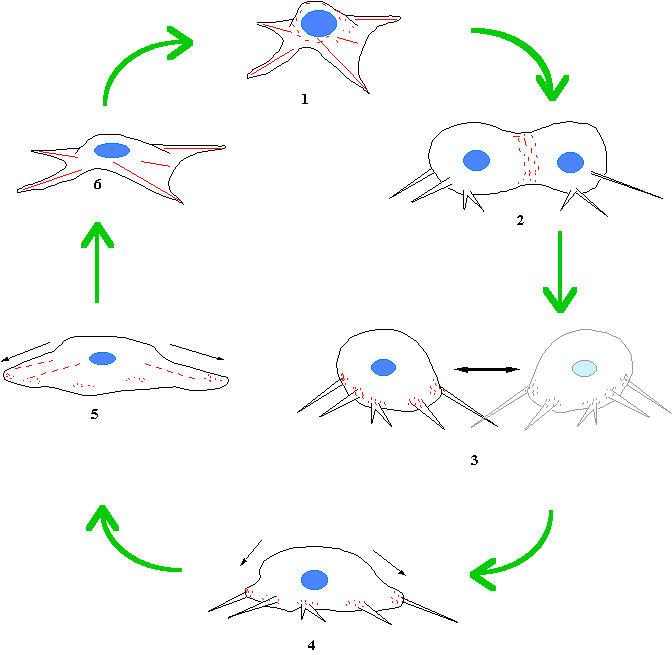
Figure 3. Myosin II is involved at each stage of
the cell cycle. (1) A fibroblastic cell at interface with mysoin II (red)
distributed in the cortex and the stress fibres maintaining cortical
tension. (2) At prophase, the cell rounds up, this probably required myosin
II as the introduction of a myosin0mimicking peptide blocks this rounding
up (Sims et al, 1992). (3) As
cytokinesis ensues, the contractile ring contracts pinching the cell in
two.(4). The myosin II filaments interact with the retration fibres as
they join the cell bodyand this pulls the cell body back down to the
substrate. (6) Normal interphase
morphology is soon re-established.
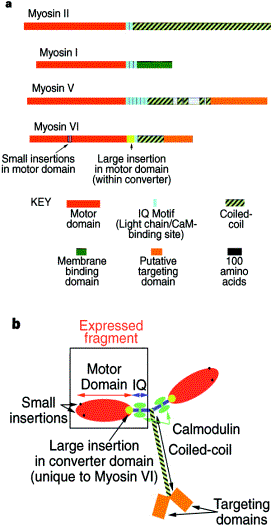
Conventional and “Unconventional” Myosins
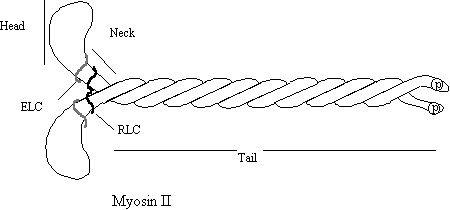
The myosins are a large superfamily of actin based motors which typically drive towards the barbed end of the actin filament (they are plus end directed motors). Conventional myosin (myosin II) is the motor that drives muscle contraction and is very common in both non-muscle but of course especially in muscle tissue. It is composed of two identical heavy chain and two pairs of light chains which assemble into the myosin II molecule (see below).
Myosin II This is the “classic” myosin found in muscle and non-muscle cells where it produces contraction (see figure 2). Knocking out the single gene encoding the myosin II heavy chain produced cells that were devoid of myosin II and established that these cells were still able to move (at reduced rates) but could not carry out cytokinesis in the normal fashion. These experiments indicate that myosin II is not absolutely required for the locomotion of Dictyostelium, but possibly "fine tunes" the process.
Two human diseases associated with
myosin II are “familial hypertrophic cardiomyopathy” (FHC) and a series of
autoimmune diseases in which bacterial infection (e.g Streptococcus pyrogenes) illicits the production of antibodies
which also cross react to cardiac myosin II causing inflammation of the heart.
|
|
The “Head” region contains the actin binding site and the ATP binding and hydrolysing site. This is where the work is done. The neck region is the regulator, containing the essential light chain (ELC) and the regulatory light chain (RLC). The RLC can be phosphorylated leading to activation of the protein complex. The neck region also acts as a lever through which the work is transmitted. The tail associated Figure 4.
with other myosins to form pi-polar “Thick filaments”. Tail -tail association is controlled by phosphorylation at the end.
Figure 5
|
|
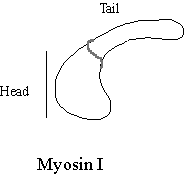
Myosin I. Not long after the discovery that myosin II existed in non-muscle cells, another single headed myosin was discovered in the soil amoeba Acanthamoeba castellanii. This myosin is very much smaller than myosin II and as it has one head, it was called “myosin I”. Myosin Is have a very short tail. Some members of the myosin I group have lipid binding sites and others have an additional actin binding site (Pollard et al, 1991). Many different myosin I types exist in cells and their localisation is distinct (Baines et al, 1992). Many myosin I isotypes are localised to membrane bound organelles and so have been hypothesised to transport the vesicles around the cell. In fact isolated Acanthamoeba myosin I supported the motility of vesicles of Acanthamoeba on actin bundles in the Nitella assay (see below). Although different myosin Is are found on, and presumably move, distinct organelles, little is yet known about how myosin I isotype recognise their cargo vesicles. A candidate myosin I docking protein Acan125 has been isolated from Acanthamoeba, it binds myosin Ic in solution via its SH3 domain and is its self localised to a subset of vesicles (Xu, Zot & Zot, 1995). The finding that a particular myosin I is associated with a particular membrane does not necessarily mean that the myosins function is to move it.
|
|
In Acanthamoeba, myosin Ic is associated with the contractile vacuole, a large organelle that is the cell’s kidney, pumping out excess water. Dobberstein and colleagues loaded an antibody to myosin Ic into the cells and found that the cells popped due to hydrostatic pressure! In this case the function of myosin Ic is clearly contractile rather than to move the vacuole (Doberstein et al, 1993).
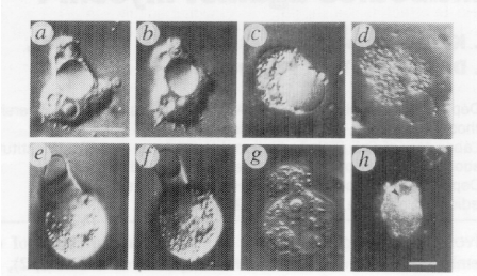
In figure 9a, an amoeba has just been loaded with the antibody and has already formed a swollen
Figure 6. contractile vacuole (in the cell centre). At subsequent times later (b, and c) it gets larger still until at d when it popped! A similar experiment is displayed on the bottom panel (e-h).
Sea urchin coelomocytes are similar to vertebrate
platelets in that they undergo a transformation in shape during the clotting
reaction (unlike platelets, they contain nuclei). As part of this reaction the cells secrete adhesive molecules
from vesicle within the cells. This
process is nocodazole (a microtubule destroying drug) insensitive, and has been
shown to result from a myosin I like motor protein tracking along polar bundles
of actin filaments, presumably with the barbed ends facing outward in the
conventional manner (D’Andrea et al,
1994). Other organelles are known to be
associated with them but the role of actin dependent motility is less
clear. Vesicles derived from golgi are
known to have a myosin-I localated on the cytoplasmic surface (Fath &
Burgess, 1993), and yet data from a number of cell and tissue types indicate
that microtubules rather than microfilaments are responsible for Golgi
structure and motility. The insect eye
adapts to light level changes by moving mitochondria and ER in opposite
directions so that the refractive index of the cell changes. This movement is actin dependent and a
myosin-I motor has been localised to the site (Sturmer & Baumann,
1998). However the polarity of the
actin filaments is correct for motility in one direction but not the other. How
the ER moves by this retro-grade actin dependent manner is not known. Myosin-1 may be responsible for the motility
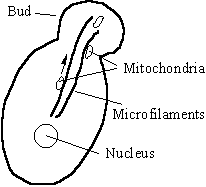
of yeast mitochondria into the daughter cell upon budding (see below).
Dictyostelium provides an excellent test for myosin function as this organism is haploid (one genome copy per cell) it is comparatively easy to knock out gene function by homologous recombination. So far 4 of the 6 (at least) myosin I genes (named myoA - myoD) have been knocked out, these cells seem healthy in the lab at least but are impaired in some aspects of motility. These knockout reveals that myoA and myoB mutants locomoted somewhat slower than wild type but that myoC and myoD mutant were as fast. MyoB and myoC were impaired in phagocytosis and all were impaired in pinocytosis. Thus there seems to be some overlap in the function of the myosin I family, but much more work is needed to establish why the cell expresses so many types.
Other Unconventional Myosins
Since the discovery of myosin I, more than a dozen other distinct myosin types have been identified (see Mermall et al, 1998 for a recent review). This has been done mainly by PCR, so knowledge of their sequence is greatly ahead of our understanding of their function. Some of these have been implicated in vesicular transport.
Myosin III Only one member of this group has been identified so far and its function is far from clear. NinaC is known only from the compound eye of Drosophila. NinaC is the most divergent member so far and has a kinase region at the N-terminus of the myosin head region. No human isoform of ninaC has yet been identified, but recently two myosin III isoforms have been identified in fish retina by PCR which will help in the search for a human homolog (Hillman et al, 1995 Invest.Ophthal.Vis Sci. abstract 36s).
Myosin IV. A high molecular weight myosin from Acanthamoeba is the only definite member of this group to date
nothing is known about its function.. No human isoform myosin IV has yet been
identified
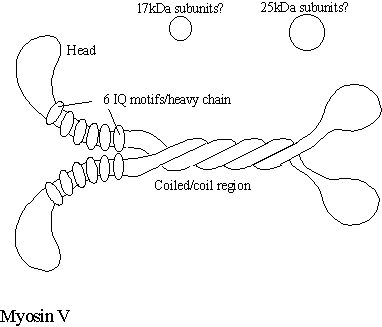
Myosin-V The gene product of the murine “Dilute” mutant turns out to be a myosin V protein. These mice die about three weeks after birth and display a variety of neurological defects, eventually leading to seizures. The name comes from the fact that the coat colour has a washed out appearance due to the failure of melanin containing vesicles to be transported into the hair. It is very likely that the dilute protein (myosin V) is involved with actin dependent transport of material down the axons of nerve cells as well as melanin vesicles in other cell type (Nascimento et al, 1997). Myosin V decorated vesicles have been found to be associated with both actin filaments and microtubule in nerve growth cones (Evans et al, 1997) leading to the speculation that such vesicles may be transported by both filament systems. In addition, the human myosin V (also called Myoxin) appears to colocalise with intermediate filaments in cultured cells. Two distinct myosin-Vs are involved with cytoplasmic inheritance of various organelles in yeast (Titus, 1997) this is discussed below.
|
|
Myosin-V consist of two heavy chains containing the myosin head and a neck with six IQ motifs each which bind a total of four calmodulin and one each of an additional 25kDa and a 17kDa light chain (Chenney et al, 1993). Presently, the function of the 25 and the 17kDa proteins are not known nor is their position relative to the myosin V molecule. Some reports suggest that myosin V binds the 8kDa light chain of dynein (Benashski et al, 1997). If this is confirmed it may mean that both motors are targeted to the same vesicle by this same light chain. The motility of myosin-V is inhibited by calcium and moves at a speed of 300nm/sec. Myosin V is a highly processive motor protein (it remains tightly bound to the filament as it moves along, even in the presence of ATP) and so seems ideally suited to its task of moving vesicles along filaments. Very recently, it has been found that Myosin V is responsible for the movement of ER along the axon of squid (Tabb et al, 1998). Ultrastructural studies in both the dilute mouse (Takagishi et al, 1996) and the dilute rat (Dekker-Ohno et al, 1996) indicate that ER is absent from the dendritic spines of Purkinje cells. As the ER is often a source of IP3 releasable calcium, this may reduce the cells excitablity. This makes sense of a human disease “Griscelli disease” in which mutations in the human myosin V gene result in ataxia, light pigmentation and a variety of immunodeficiencies and neurological based symptoms (Hurvitz et al, 1993).
 Myosin-VI Mutation in the mouse myosin VI gene result in the Snell’s watzer phenotype resulting from
vesibular disfunction. The gene is
expressed in other tissues however. It
is thought that myosin VI in the stereocilia may play a role in stabilizing the
structure which is under mechanical perturbation with every noise. Myosin
VI. These mice show an inner ear
degeneration starting at the organ of corti but with complete loss of the
sensory epithelium by six weeks. No
human disease of this nature has been discovered as yet.
Myosin-VI Mutation in the mouse myosin VI gene result in the Snell’s watzer phenotype resulting from
vesibular disfunction. The gene is
expressed in other tissues however. It
is thought that myosin VI in the stereocilia may play a role in stabilizing the
structure which is under mechanical perturbation with every noise. Myosin
VI. These mice show an inner ear
degeneration starting at the organ of corti but with complete loss of the
sensory epithelium by six weeks. No
human disease of this nature has been discovered as yet.





Myosin-VII Mutations in this gene too cause deafness but an additional burden of blindness. In the mouse, mutaution of this gene produces the Shaker 1 phenotype. The human disease associated with myosin VII is called Usher’s syndrome (more about this in the next session).
Myosin XV The latest member of the myosin super-family was reported to be associated with human deafness. This gene is now known to be a homolog of the mouse mutant shaker 2 (Probst et al, 1998; Wang et al, 1998).
Yeast Cytoplasmic Inheritance and Myosins
Many organelles cannot be synthesised by cells without duplication from pre-existing organelles. This makes it imperative that prior to scission at cytokinesis, the various organelles are equally distributed between the daughter cells. In the yeast Saccharomyces cerevisiae, mitochondria are transported from the mother cell to the daughter cell as the daughter cell buds off (Simon et al, 1997). The analysis of mutant deficient in mitochondrial inheritance experiments implicates a number of proteins in this process in yeast
|
Figure 10 |
Gene Gene product
ACT1 Yeast actin
TPM1 Tropomyosin. (messed up actin).
MDM1 An intermediate filament type protein
MDM2 A fatty acid desaturase
MMM1 Mitochondria outer membrane protein
MDM10 Mitochondria outer membrane protein
Actin filaments stretch from the bud site into the mother cell. Some of the mutants (TPM1, ACT1) fail to produce this bundle, whereas others (MMM1, MDM10, ARP) probably fail to target the motor to the mitochondria. Although the motor has not been definitively identified, actin dependent motor activity has been isolated from the surface of yeast mitochondria (Simon et al, 1995). This activity was seen in yeast lacking, Myo1, Myo2, Myo3 and Myo4 and as the yeast genome has now been completed we know that only one identifiable myosin gene (Myo5) has still to be accounted for (Brown, 1997). Myo5 despite its name is a yeast myosin-I. The cytoplasmic inheritance of other vesicles and organelles has been found to depend on several myosin types, notably Myosin V (Titus, 1997). The chitin producing enzyme containing vacuoles are known to be transported by myo2p, one of the two types of Myosin-V in yeast, while the other myosin-V, myo4p is responsible for taking a complex including the mRNA for ASH to the bud site.
|
Figure 11 |
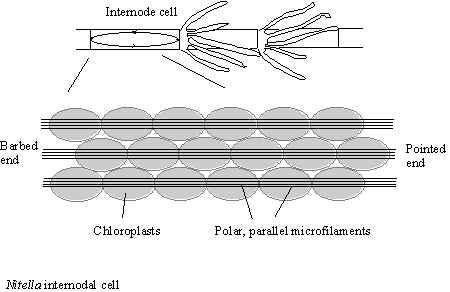
Nitella , a bioassay for
myosin activity
The cytoplasmic content of the internodal cells of the “Giant” algae Nitella are in constant unidirectional motion. This is due to the interaction of a myosin coating the organelles in the cytoplasm interacting with a series of actin filament bundles which lie on top of the chloroplasts, lining the cell wall (see figure 11)
This unique arrangement can be made use of as it is so large, the actin bundle surface can be exposed by surgery of the cells. The cell wall can be cut open and pinned back and the experimenter can then add the test solution and look for motility. This assay was first due for myosin-I to prove that the organelles from Acanthamoeba were decorated with myosin-I that allowed their transport. This motility was shown to be due to myosin-I, as specific antibodies inhibited the flow of the organelles (Adams & Pollard, 1991).
References
Adams,
R.A. and Pollard, T.D. (1989) Nature 340;
565-588.
Baines,
I.C., Brzeska, H., Korn, E.D. (1992). Differential localization of Acanthamoeba myosin I isoforms. J.Cell Biol. 119; 1193-1203.
Benashski,
S.E., Harrison, A., Patel-King, R.S., and King S.M.(1997) J.Biol.Chem. 272; 20929-
Bloom, G.S. and Goldstein,
L.S.B. (1998) Cruising along microtubule highways: How membranes move through the secretory pathway. J.Cell
Biol. 140; 1277-1280.
Brown,
S.S. (1997) Myosins in yeast Curr.Op.Cell
Biol. 9; 44-48.
Chenney,
et al (1993) Brain myosin-V is a
two-headed unconventional myosin with motor activity. Cell 75; 13-23.
D’Andrea, L., Danon, M.A.,
Sgourdas, G.P. and Bonder, E.M. (1994) Identification of coelomocyte
unconventional myosin and its association with in vivo particle/vesicle
motility. J.Cell Sci. 107;
2081-2094.
Dekker-Ohno
et al, (1996) Endoplasmic reticulum is missing in dendritic spines of Purkinje
cells of the araxic mutant rat. Brain
Res. 714, 226-230.
Doberstein,
S.K., et al (1993). Inhibition of contractile vacuole function in vivo by antibodies against myosin
I. Nature
365; 841-843.
Evans, L.L., Hammer, J., and
Bridgeman, P.C. (1997) Subcellular localization of myosin V in nerve growth
cones and outgrowth from dilute-lethal
neurons. J.Cell Sci. 110;
439-449.
Fath, K.R. and Burgess, D.R.
(1993) Golgi-derived vesicles from developing epithelial cells bind actin
filaments and possess myosin-I as a cytoplasmically oriented peripheral
membrane protein. J.Cell Biol. 120;
117-127.
Hurvitz,
H., et al, (1993) A kindred with
Griscelli disease: Spectrum of
neurological involvement. Eur.J.
Pediatrics 152; 402-405.
Pollard,
T.D., Doberstein, S.K., Zot, H.G. (1991) Myosin I. Ann.Rev.Physiol. 53;
653-681.
Lantz, V.A. And Miller, K.G.
(1998) A class VI unconventional myosin is associated with a homologue of a
microtubule-binding protein, cytoplasmic linker protein-170, in neurons and at
the posterior pole of Drosophila
embryos. J.Cell Biol. 140,
897-910.
Lillie,
S.H., and Brown, S.S. (1998). Smy1p, a kinesin-related protein that does not
require microtubules. J.Cell Biol. 140;
873-883.
Maciver,
S.K. (1996). Myosin II function in non-muscle cells. BioEssays 18; 179-182.
Mermall,
V., Post, P, Mooseker, M.S. (1998).
Unconventional myosins in cell movement, membrane traffic, and signal
transduction. Science 279; 527-533.
Miller, D.P., Scordilis,
S.P., and Hepler, P.K. (1995).
Identifiaction and localization of three classes of myosins in pollen
tubes of Lillium longiflorum and Nicotiana alata. J.Cell
Sci. 108; 2549-2653.
Nascimento, A.A.C., et al (1997) Subcellular localization of
myosin Vin the B16 melanoma cells, a wild type cell line for the dilute gene. Mol.Biol.Cell 8;
1971-1988.
Probst, F.J. et al, (1999) Correction of deafness in
shaker-2 mice by an unconventional myosin in a BAC transgene. Science 280; 1444-1447.
Ostap
& Pollard. “Overlapping functions of myosin-1 isoforms?” J.Cell Biol. 133, 221.April 1996
Richard,
J.E. and Kries, T.E. (1996) CLIPs for organelle-microtubule interactions Trends Cell Biol. 6; 178-183.
Simon, V.R., Karmon, S.L. and
Pon, L.A. (1997). Mitochondrial inheritance: Cell cycle and
actin filament dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell
Motl.Cytoskeleton. 37; 199-210.
Sims, J.R. (1992)Altering the
cellular mechanical force balance results in integral changes in cell,
cytoskeletal and nuclear shape. J.Cell
Sci.103; 1215-1222.
Sturmer, K. and Baumann, O.
(1998). Immunolocalization of a putative unconventional myosin on the surface
of motile mitochondria in locust photoreceptors. Cell Tissue Res. 292; 219-227.
Tabb,
J.S., et al(1998) Transoprt of ER
vesicles on actin filaments in neurons by myosin V. J.Cell Sci. 111; 3221-3234.
Takagishi
et al, (1996) The dilute-lethal (dI)
gene attacks a Ca2+ store in dendritic spine of Purkinje cells in mice Neurosci Letters. 215, 169-172
Titus, M.A. (1997) Motor proteins: Myosin V – the
mutli-purpose transport motor. Curr.Biol. 7; 301-304.
Wang, A. et al. (1998) Association of unconventional
myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280; 1447-1451
Wells, A.L.et al, (1999). Myosin VI is an
actin-based motor that moves backwards. Nature
401; 505-508.
Wright & Jackson, “Myosin
diversity and disease” Trends in Genetics
12, (6). 206-209.
Some useful Related Websites
Tutorial on Actin & Myosin Structures:- http://chon.bch.ed.ac.uk/paul/TEACHING/HONS/MUSCLE/
Myosin head structure http://salus.med.uvm.edu/~guilford/myosin.html
Hereditary Hearing Loss homepage http://dnalab-www.uia.ac.be/dnalab/hhh
General Myosin webpage http://www.mrc-lmb.cam.ac.uk/myosin/myosin.html