|
Chemotaxis |
|
Page updated 13/11/02 |
| Amoeba
feed on other protist, algae and bacteria. They must be able
to adapt to the temporary absence of suitable prey, for example by
entering resting stages such as cysts, but it is obviously an advantage
to be able to "smell out" prey items and craw toward the
source. This ability is chemotaxis. It is likely that all
amoebae have this ability since it would confer such a huge advantage to
these organisms, indeed chemotaxis has been demonstrated in Amoeba
proteus, Acanthamoeba, Naegleria and Entamoeba.
The best studied chemotactic amoeboid organism however is Dictyostelium
discoideum. Dictyostelium is chemotactic for bacteria,
but has been extensively studied for its ability to climb gradients of
cAMP, a signalling molecule involved in the development of the slug
(figure 1). The
term "Chemotaxis" was first coined by a W. Pfeffer in 1884 to
describe the attraction of fern sperm to the ova, but since then the
phenomenon has been described in bacteria and many eukaryotic cells in
many different situations. Specialised
cells within metazoans have retained the ability to crawl toward
bacteria in order to eliminate them from the body and the machinery is
very similar to that used by primitive eukaryotes to find bacteria for
food. Much of what we know
about chemotaxis has been learned from studying the slime mould Dictyostelium
discoideum, and comparing this to our own neutrophils, the white
blood cells that detect and consume invading bacteria in our bodies.
Neutrophils are end differentiated and largely non-biosynthetic
cells which means that we cannot use the usual molecular biological
tools that we would other wise use, such as gene knockout, transient
transfection, or expression of GFP-labelled proteins.
Fortunately, Dictyostelium can be used for all such
studies. An understanding
of neutrophils chemotaxis is of obvious importance for the treatment of
human disease and therapeutic intervention of these processes has
resulted. Whereas
chemotaxis is the sensing of a chemoattractant gradient and climbing it
cells can also accumulate by chemokinesis.
Chemokinesis is an activity that increases the overall speed of
locomotion. In
principle a cell be perceive gradients by a number of distinct
mechanisms (Figure 1). These hypothesis have been offered in various guises by a
number of authors. |
 |
Figure 1. How cells may
perceive chemo-attractant gradients (after Lackie, 1986).
The temporal model assumes that the amoeba measures and
"remembers" the chemo-attractant concentration at time zero
and compares this to a second reading at some time point later as the
cell crawls. In this way
the amoeba gets clues like that particularly annoying party game
"getting warmer" / " getting colder" (Arghhh!!).
The spatial model assumes that the cell is able to read
the concentration across the span of the cell so that it can compare the
number of chemoattractant molecules. A related model is the pseudospatial model, where the
cell tests concentrations by sending out pseudopods (P1 and P2)
at various points to sense where the highest concentrations lie.
The temporo-spatial model, detects waves of chemo-attractants
coming toward the cell from a particular direction.
Note that this last mechanism could not read a static gradient.
A huge amount of work has been expended trying to work out which
(if any) of these hypotheses is correct. |
|
The
distribution of cAMP receptors on Dictyostelium has been
determined by replacing the gene with a gene consisting of the receptor
fused to green fluorescent protein (GFP –see below).
These studies (Xiao et al, 1997) indicate that there is no
particular concentration of receptors within pseudopods as expected from
the spatial, pseudospatial or temporo-spatial models.
However we cannot discount these models on this basis since they
could all function in principle without concentrated receptors (it would
just work better). The temporo-spatial model cannot be valid for all cases as it
is well established that cells can chemotax in stable gradients of
attractions.
The
Boyden chamber.
Two chambers are separated by a filter through which cells
migrate (page 7, figure15). Chemotactic gradients can be set up by placing different
concentrations of the putative chemo-attractant in the upper and lower
chambers. The advantage of
the Boyden chamber is that is can discriminate between chemo-kinetic and
chemotactic influences. The
use of this chamber requires that the cells under test have to move in
three dimensions (most do) and to squeeze between the pore size of the
particular filter. Filters
are available in different average pore sizes, so this latter point is
seldom a problem. The Boyden chamber is reproducible and the chemokinetic,
chemotactic response easy to quantify. |
 |
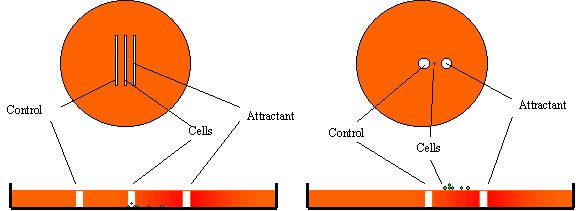
| Figure
2. The Boyden Chamber. The
main advantage is that it can detect chemokinetic effects as well as
chemotactic effects. The Checkerboard assay (right) is a way to organise the
Boyden chamber experiment. |
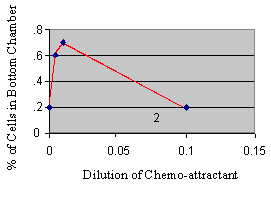 |
Figure
3.
The
checkerboard assay can be used to differential chemokinesis from
chemokinesis.
If attractants are added to both chambers at various
concentrations, the relationship of migration to attractant can be
plotted.
In this example, the attractant is both a chemokinetic and a
chemoattractant. |
 |
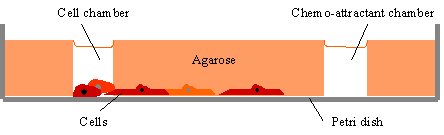
| Figure
4. The under agarose
method (left) versus the over agarose method.
The under agarose method (Laevsky & Knecht, 2001 ) is simple
and can be used for a variety of cell types.
Chemo-attractant gradients are set up as the attractant diffuses
from the trough into the agarose. The presence of the agarose stabilizes the gradient that
might otherwise be dispersed or changed by thermal motion or minor
agitations. An advantage of
the various under agarose methods is that it is possible to set up
multiple gradients of various chemo-attractants to study their effect on
cell behaviour simultaneously (Heit et al, 2002). |
| Figure
5.
A stable gradient can be created by placing the putative
attractant in one well and the cells are then placed in a second well. |
 |
|
Chemotaxis
in Development
Mammalian
development begins with the meeting and fusion of the gametes, the
female egg gives signals and the male sperm comes swimming (setting the
pattern for life?). Chemotaxis
helps the sperm find the egg in humans (Eisenbach & Tur-Kaspa,
1994), and in algae where a chemo-attractant increases the turning angle
of the sperm cell so that it spirals in toward the egg like a captured
moon. Development involves
mass movements of cells.
|
|
|
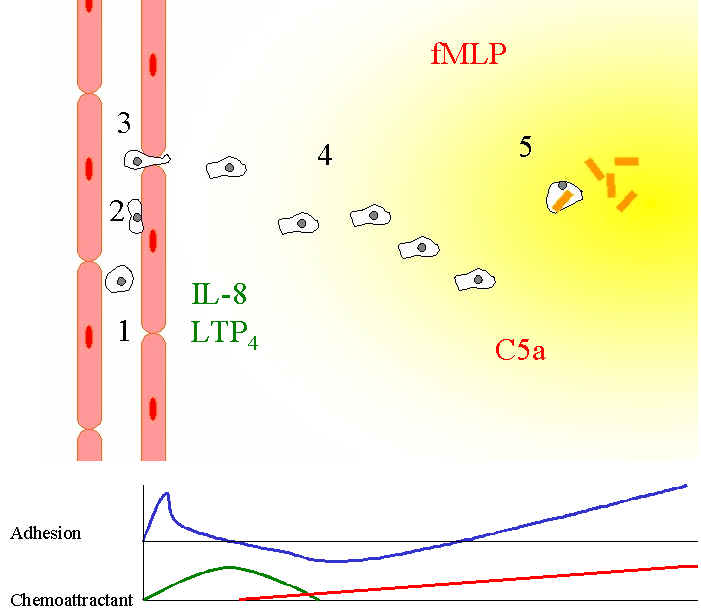
| Figure
6. Neutrophil
migration into inflammatory regions.
(1). Neutrophils in the microvascular vessels circulate
passively in the blood.
At sites close to inflammation (2), the endothelial cells
alter their charge in response to interleukins such as IL-8, so that now
neutrophils can stick.
(3). Neutrophils now crawl along the "-dimensional
surface of the vessel and then squeeze between the endothelial cells.
(4) Neutrophils can now invade the 3-dimensional space and
begin to detect the fMLP and/or C5a gradient at the same time as a
negative IL-8, LTP4 gradient.
It has been suggested (Heit et al, 2002), that such an
arrangement is more stimulatory.
(5) Neutrophils arrive at the site of infection drawn by
the gradient and now consume the bacteria by phagocytosis.
Eventually the neutrophils die full of now dead bacteria and
accumulate as pus.
The top line in the graph represents adhesive forces which peak at the endothelial surface, drop after that as
the neutrophils crawl in the 3-dimension matrix. Adhesion then increases with fMLP/C5a gradient.
The lower graph represents the expected gradient of the various
classes of chemo-attractants (after Lackie).
|
|
Transduction
of Chemo-attractant Signals
Most
of what is known about the signalling cascade has been gleaned from Dictyostelium,
but recent contributions are available from knock out mouse models.
From the top, chemotaxis requires the chemotactic receptor,
heterotrimeric G-proteins,
Whereas the distribution of chemotactic receptors is uniform across the
cell (Xiao et al, 1997), other signalling molecules further down
the cascade are found to have a polarized distribution.
Mammalian PLCg
become localised to the leading edge in a PI3-Kinase dependent manner (Piccola
et al, 2002). PLCg
contains a PH domain that binds PtdIns-3,4,5-P3, a product of
PI3-Kinase, and so its probable that the PLCg
localization
is downstream of PI3-Kinase activity.
The bg
subunit of the activated hetero-trimeric G-proteins are weakly polarised
fashion with concentrations at the leading edge (Jin et al,
2000). Another protein
involved in chemotactic pathway AKT (protein kinase B), also contain PH
domains and are also localized to the leading edge of cells (neutrophils)(Servant
et al, 2000). Surprisingly,
it has recently been reported that in mouse neutrophils, PLCs are not
required for chemotaxis but are involved in priming the superoxide burst
(Li et al, 2000). Dictyostelium
too seems to chemotax in the absence of PLC (Drayer et al, 1994). |
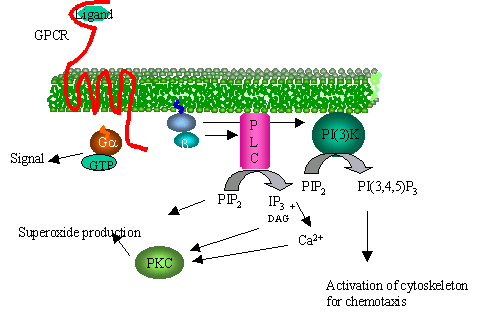 |
Figure
7.
G-protein linked chemo-attractant receptor dissociates trimeric
G-protein and the b,g
subunit activates both PLC and PI(3)-kinase.
The products of PI(3)-kinase are short-lived messengers that bind
a number of targets leading to their activation.
The activation of PLC is important for activation of the
superoxide burst after phagocytosis in neutrophils but not important in
chemotaxis its self.
Mice bred without PI(3)-kinase however have a reduced capacity to
chemotax. |
| References:-
Key, T. A.,
Foutz, T. D., Gurevich, V. V., Sklar, L. A. & Prossnitz, E. R.
(2003) N-Formyl Peptide Receptor Phosphorylation Domains Differentially
Regulate Arrestin and Agonist Affinity. J. Biol. Chem. 278,
4041-4047.
|
|
