|
A
family of actin binding proteins typified by smooth muscle specific protein which binds G and F-actin.
Calponins are abundant proteins in smooth muscle and are the products of
three distinct genes. The most abundant form is a-calponin
(h1 or basic) is 292 amino acids (34kDa), b-calponin
is 252 amino-acids in length have a deletion of amino-acids 217-256 of
the same gene as a-calponin.
Two other genes encode neutral calponin (h2 calponin) and acidic
calponin are expressed at lower levels in smooth and non-muscle cells. Other more widely expressed
calponin members include SM22, and transgelin.
Calponin ironically has given its name to the CH or Calponin Homology domain
present in many ABPs, despite the fact that calponin does not seem to bind actin through this
region! (Gimona
& Mital, 1998),
but instead binds lipids (Bogatcheva
& Busev, 1995; Fujii et al, 1995).
The principle actin binding site is believed to exist (Mezgueldi
et al, 1992; )
at a distinct region between the CH domain and the calponin repeats.
Calponin is
an abundant heat stable, basic protein in smooth muscle where it is
present as a 34kDa species. In other tissues a more acidic isoform
(acidic CaP) is present it is calcium independent but binds calmodulin in a
sensitive manner. Binding
of Ca2+/calmodulin inhibits actin binding.
Phosphorylation also inhibits actin binding. Calponin binds
tropomyosin another actin binding protein. Calponin binds many
other proteins, many of which are also actin binding proteins (table
1) in addition to phospholipids (Bogatcheva
& Busev, 1995).
Calponin
Structure
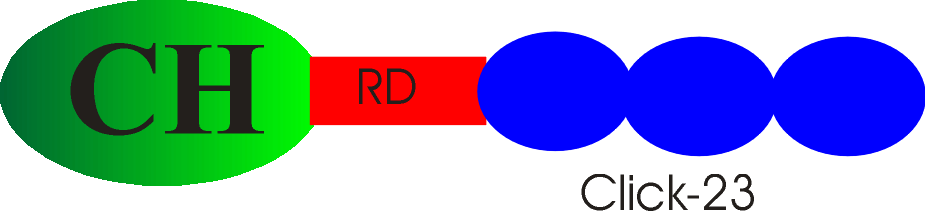 |
Figure
1. The
domain structure of calponin |
The
most celebrated feature of calponin is the so called Calponin Homology
(CH) domain as mentioned previously. Calponins have just one
single CH domain. Each CH domain is about 100 amino-acids in
length and in the many actin binding proteins that are known to
bind actin through CH domains, these are present as two dissimilar
domains. When present singly as in calponin itself, it seems that
the CH domain is insufficient to bind actin (like trying to pick up a
pint with one hand while wearing boxing gloves!?) (Gimona
& Mital, 1998).
The fact that calponin does not bind actin through the CH domain is consistent
with the observation that calponin competes neither with
filamin (Panesenko
& Gusev, 2001)
or a-actinin
for actin binding (Leinweber
et al, 1999;
Panesenko
& Gusev, 2001),
both of which bind through twin CH domains. The single CH domain in
calponin is known to bind Ca2+/calmodulin, ERK, tropomyosin (Winder
& Walsh, 1993).
The structure of the single CH domain in calponin itself has overall
similarity with the CH domains of those naturally arranged in tandem,
such as in utrophin (Keep
et al, 1999)
or fimbrin (Goldsmith
et al, 1997).
The single calponin CH domain however has differs significantly possibly
explaining why the single CH domain cannot bind actin (Bramham
et al, 2002).
The
regulatory domain (RD) contains the actin binding domain which has reported
homology to troponin I (Takahashi
et al, 1991). There are also sites for
phosphorylation by PKC (Ser 175) and by CaM/kinase II.
Click-23
The
calponin repeat domain at the C terminus is proposed to form WD40
like structures through which many interactions with other proteins is
made. Secondary actin binding site may exist in the calponin repeats (Bonet-Kerrache
& Mornet,1995),
very possibly right next to the main site, at the start of repeat
1. Kolakowski
et al, 1995;
Tang et al, 1997).
An actin binding site is reported to exist within the
repeats. Recently, (Kranewitter
et al, 2001)
it has been shown that Unc-87 is an actin filament bundling protein
composed of seven calponin repeats, these authors have dubbed the
calponin repeat CLICK-23 (from the fact that the repeat is 23amino-acids
in length and is Calponin Like).
| Protein |
Function |
Binding
site on Calponin |
References |
| a-actinin |
Strengthens the a-actinin-actin
complex |
|
Leinweber et al, 1999a |
| Actin |
Modulates
actin: myosin II ATPase activity. |
Regulatory domain and |
Mezgueldi,et
al, 1992;
1995 |
| Caldesmon |
Myosin II binding/regulation |
|
|
| Caltropin |
Myosin II binding/regulating |
Regulatory domain |
Wills et al, 1994 |
| Calmodulin |
Inhibits
actin binding to regulatory domain in Ca2+. |
Two sites, one in the CH
(1-52) domain, the other in the RD (145-163) |
Winder
et al, 1993; |
| ERK |
Extra-cellular
regulatory kinase |
CH
domain |
Leinweber et al, 1999b |
| Hsp70 |
Heat shock protein |
7-144 |
Bogatcheva
et al, 1999;
Ma et al, 2000 |
| Myosin II |
Stimulated
myosin II ATPase activity |
146-176 |
Szymanski
et al,1997; Lin et al,1993 |
| Protein kinase C |
Activates kinase activity |
Calponin
repeats (WD40) |
Leinweber et al, 2000 |
| Tropomyosin |
Actin stability and myosin
II regulation . |
Two sites, one in the CH
(1-52) domain, the other in the RD (145-163) |
Takahashi
et al, 1988; Vancompernolle
et al, 1990;Childs et al,
1992; Mezgueldi,et
al, 1995. |
| Tubulin |
Microtubule binding and
bundling? May also connect other cytoskeletal elements
e.g. actin to microtubules. |
Two sites, one in the actin
and Calmodulin binding region 145-182, the other in the C
terminal repeat region 183-292 |
Fuji et al, 1997;
Fattoum et al, 2003 |
|
|
|
|
| Table
1 Calponin-Binding proteins |
Calponin
Function
The
function of the calponins has long been assumed to be to regulate the
interact between actin filaments and myosin II in smooth muscle
contraction and analogous interactions in non-muscle cells (Takahashi
et al, 1986;
Winder
& Walsh 1993;
El-Mezgueldi 1996;
Gusev 2001).
The consensus has been that h1 calponin inhibits the actin-activated
ATPase of myosin II thereby regulating the interaction. However,
the h1 gene has been knocked out in a mouse model (Yoshikawa
et al, 1998)
and the resulting phenotype has been a surprise. The h1 calponin
was found not to be compensated by up-regulation of other calponins and
the most obvious effect was on the bone (as is the knock out of another
ABP ()). h1 calponin is highly expressed in osteoblasts and
may act as a negative regulator of the bone making process.
Analysis of the knock out mouse (Matthew
et al, 2000),
revealed that compared with wild type mouse there was no difference in
Ca2+ sensitisation pathways but there was a clear increase in
unloaded shortening velocity in K.O. mice muscle and that this could be
overcome by adding back h1 calponin to the tissue. These findings
are consistent with a role for calponin in the regulation of
actin:myosin interaction but the study also showed a drastic reduction
in the expression of actin (about 50%).
References:-
Applegate,
D., Feng, W., Grren, R. S. & Taubman, M. B. (1994) Cloning and
expression of a novel acidic calponin isoform from rat vacular smooth
muscle., J.Biol.Chem. 269, 10683-10690.
Bańuelos, S., Saraste, M. & DJinovi´c
Carugo, K. (1998) Structural comparisons of calponin homology domains:
implications for actin binding. Curr. Biol. 6, 1419-1431.
Bárány, K., Polyák, E. & Bárány, M.
(1992) Involvement of calponin and caldesmon in sustained contraction of
arterial smooth muscle. BBRC. 187, 847-852.
Bartegi, A., Roustan, C., Kassab, R. &
Fattoum, A. (1999) Fluorescence studies of the carboxyl-terminal domain
of smooth muscle calponin. Eur.J.Biochem. 262, 335-341.
Bramham, J., Hodgkinson, J. L., Smith,
B. O., Uhrin, D., Barlow, P. N. & Winder, S. J. (2002) Solution
structure of the calponin CH domain and fitting to the 3D-helical
reconstruction of F-actin:calponin. Structure. 10, 249-258.
Bogatcheva, N. V., Ma, Y. S., Urosev, D. &
Gusev, N. B. (1999) Localization of calponin binding sites in the
structure of 90 kDa heat shock protein (Hsp90)., FEBS letters. TBA,
TBA.
Bogatcheva, N. V. & Busev, N. B. (1995)
Interaction of smooth muscle calponin with phospholipids. FEBS
letters. 371, 123-126.
Bonet-Kerrache, A. & Mornet, D. (1995)
Importance of the C-terminal part of actin in interactions with
calponin.
BBRC. 206, 127-132.
Childs, T. J., Watson, M. H., Novy, R. E., Lin,
J. J.-C. & Mak, A. S. (1992) Calponin and tropomyosin interactions. BBA. 1121, 41-46.
Danninger,
C. & Gimona, M. (2000). "Live dynamics of GFP-calponin: isoform-specific
modulation of the actin cytoskeleton and autoregulation by C-terminal
sequences. J.Cell Sci. 113, 3725-3736.
Djinovic
Carugo, K., Bańuelos, S. & Saraste, M. (1997) Crystal structure of
a calponin homology domain. Nature Struct. Biol. 4, 175-179
EL-Mezgueldi, M. (1996) Calponin. Int. J.
Biochem.Cell Biol. 28, 1185-1189.
EL-Mezgueldi, M. & Marston, S. B. (1996)
The effects of smooth muscle calponin on the strong and weak myosin
binding sites of F-actin. J.Biol.Chem. 271, 28161-28167.
Fattoum, A., Roustan, C., Smycznski,
C., Der Terrossian, E. & Kassab, R. (2003) Mapping the microtubule
binding regions of calponin. Biochemistry. 42, 1274-1282.
Fu,
Y., Liu, H. W., Forsythe, S. M., Kogut, P., McConville, J. F., Halayko,
A. J., Camoretti-Mercado, B. & Solway, J. (2000) Mutagenesis
analysis of human SM22: characterization of actin binding. J. Appl.
Physiol. 89, 1985-1990.
Fuji,
T., Hiromori, T., Hamamoto, M. & Suzuki, T. (1997) Interaction of
chicken gizzard smooth muscle calponin with brain microtubules. J.Biochem.
122, 344-351.
Fujii, T., Yamana, K., Ogoma, Y. & Kondo,
Y. (1995) Interaction of calponin with phospholipids. J.Biochem. 117,
999-1003.
Fujii, T., Oomatsuzawa, A., Kuzumaki, N. &
Kondo, Y. (1994) Calcium-dependent regulation of smooth muscle calponin
by S100. J.Biochem. 116, 121-127.
Gimona,
M. & Mital, R. (1998). The single CH domain of calponin is
neither sufficient nor necessary for F-actin binding. J.Cell
Sci. 111, 1813-1821.
Goldsmith,
S. C., Pokala, N., Shen, W., Federov, A. A., Matsudaira, P. & Almo,
S. A. (1997) The structure of an actin-cross-linking domain from human
fimbrin. Nature Struct. Biol. 4, 708-712.
Gong,
B.-J., Mabuchi, K., Takahashi, K., Nadal-Ginard, B. & Tao, T. (1993)
Characterization of wild type and mutant chicken gizzard a
calponin expressed in E.coli., J.Biochem. 114, 453-456.
Gusev,
N. B. (2001) Some properties of caldesmon and calponin and the
participation of these proteins in regulation of smooth muscle
contraction and cytoskeleton formation., Biochemistry (Moscow). 66,
1112-1121.
Haeberle,
J. R. (1994) Calponin decreases the rate of cross-bridge cycling and
increases maximum force production by smooth muscle myosin in an in
vitro motility assay., J.Biol.Chem. 269, 12424-12431.
Ichikawa, K., Ito, M., Okubo, S., Konishi, T.,
Nakano, T., Mino, T., Nakamura, F., Naka, M. & Tanaka, T. (1993)
Calponin phosphatase from smooth muscle: a possible role of type 1
protein phosphatase in smooth muscle relaxation., BBRC. 193,
827-833.
Kolakowski, J., Makuch, R., Stepowski, D. &
Dabrowska, R. (1995) Interaction of calponin with actin and its
functional implications., Biochem.J. 306, 199-204.
Kranewitter, W. J., Ylanne, J.
& Gimona, M. (2001) UNC-87 is an actin-bundling protein., J.Biol.Chem.
276, 6306-6312.
Keep, N. H., Norwood, F. L. M., Moores, C. A.,
Winder, S. J. & Kendrick-Jones, J. (1999) The 2.0Ĺ structure of the
second calponin homology domain from the actin-binding region of the
dystrophin homologue utrophin, J.Mol.Biol. 285, 1257-1264.
Leinweber, B., Tang, Z. X., Stafford, W. F.
& Chalovich, J. M. (1999a) Calponin interaction with a-actinin-actin:
evidence for a structural role for calponin., Biophys. J. 77,
3208-3217.
Leinweber, B., Parissenti, A. M.,
Gallant, C., Gangopadhyay, S. S., Kirwan-Rhude, A., Leavis, P. C. &
Morgan, K. G. (2000) Regulation of protein kinase C by the cytoskeletal
protein calponin., J.Biol.Chem. 275, 40329-40336.
Leinweber, B. D., Leavis, P. C.,
Grabarek, Z., Wang, C.-L. A. & Morgan, K. G. (1999b) Extracellular
regulated kinase (ERK) interaction with actin and the calponin homology
(CH) domain of actin-binding proteins., Biochem.J. 344, 117-123.
Leinweber, B., Parissenti, A. M.,
Gallant, C., Gangopadhyay, S. S., Kirwan-Rhude, A., Leavis, P. C. &
Morgan, K. G. (2000) Regulation of protein kinase C by the cytoskeletal
protein calponin., J.Biol.Chem. 275, 40329-40336.
Lin, Y., Ye, L.-H., Ishikawa, R., Fujita, K.
& Kohama, K. (1993) Stimulatory effect of calponin on myosin ATPase
activity., J.Biochem. 113, 643-645.
Lehman, W. (1991) Calponin and the composition
of smooth muscle thin filaments., J.Muscle Res.Cell Mot. 12,
221-224.
Matthew, J. D., Khromov, A. S.,
McDuffie, M. J., Somlyo, A. V., Somlyo, A. P., Taniguchi, S. &
Takahashi, K. (2000) Contractile properties and proteins of smooth
muscles of a calponin knockout mouse., J.Physiol. 529.3, 811-824.
Mezgueldi, M., Fattoum, A., Derancourt, J.
& Kassab, R. (1992) Mapping of the functional domains in the
amino-terminal region of calponin., J.Biol.Chem. 267,
15943-15951.
Mezgueldi, M., Mendre, C., Calas, B., Kassab,
R. & Fattoum, A. (1995) Characterization of the regulatory domain of
gizzard calponin, J. Biol. Chem. 270, 8867-8876.
Ma, Y.-S., Bogatcheva, N. V. &
Gusev, N. B. (2000) Heat shock protein (hsp90) interacts with smooth
muscle calponin and affects calponin-binding to actin., BBA. 1476,
300-310.
Miki, M., Walsh, M. P. & Hartshorne, D. J.
(1992) The mechanism of inhibition of the actin-activated myosin
MgATPase by calponin., BBRC. 187, 867-871.
Nakamura, F., Mino, T., Yamamoto, J., Naka, M.
& Tanaka, T. (1993) Identification of the regulatory site in smooth
muscle calponin that is phosphorylated by protein kinase C., J.Biol.Chem.
268, 6194-6201.
Nishida, W., Kitami, Y. & Hiwada, K. (1993)
cDNA cloning and mRNA expression of calponin and SM22 in rat aorta
smooth muscle cells., Gene. 130, 297-302.
North, A. J., Gimona, M., Cross, R. A. &
Small, J. V. (1994) Calponin is localised in both the contractile
apparatus and the cytoskeleton of smooth muscle cells., J.Cell Sci.
107, 437-444.
Noda, S., Ito, M., Watanabe, S., Takahashi, K.
& Maruyama, K. (1992) Conformational changes of actin induced by
calponin., BBRC. 185, 481-487.
Panasenko, O. O. & Gusev, N. B. (2001)
Mutual effects of a-actinin,
calponin and filamin on actin binding, Biochim.Biophys.Acta. 1544,
393-405.
Stradal, T., Kranewitter, W., Winder, S.J.
& Gimona, M. (1998) CH domains revisited. FEBS letters 431,
134-137.
Stafford III, W. F., Mabuchi, K., Takahashi, K.
& Tao, T. (1995) Physical characterization of calponin., J.Biol.Chem.
270, 10576-10579.
Shirinsky, V. P., Biryukov, K. G., Mettasch, J.
H. & Sellers, J. R. (1992) Inhibition of the relative movement of
actin and myosin by caldesmon and calponin., J.Biol.Chem. 267,
15886-15892.
Strasser, P., Gimona, M., Moessler, H., Herzog,
M. & Small, J. V. (1993) Mammalian calponin. Identification and
expression of genetic variants., FEBS letters. 330, 13-18.
Szymanski, P. T., Grabarek, Z. &
Tao, T. (1997) Correlation between calponin and myosin subfragment 1
binding to F-actin and ATPase inhibition., Biochem. J. 321,
519-523.
Szymanski, P. T. & Tao, T. (1993)
Interaction between calponin and smooth muscle myosin., FEBS letters.
331, 256-259.
Takahashi, K., Abe, M., Hiwada, K. & Kokubu,
T. (1988) A novel troponinT-like protein (calponin) in vascular smooth
muscle: interaction with tropomyosin paracrystals., J.Hypertension. 6,
S40-S43.
Takahashi, K. & Nadal-Ginard, B. (1991) J.Biol.Chem.
266, 13284-13288.
Tang, J. X., Szymanski, P. T., Janmey, P. A.
& Tao, T. (1997) Electrostatic effects of smooth muscle calponin on
actin assembly, Eur.J. Biochem. 247, 432-440.
Trabelsi-Terzidis, H., Fattoum, A., Represa,
A., Dessi, F., Ben-ari, Y. & Der Terrossian, E. (1995) Expression of
an acidic isoform of calponin in rat brain: Western blots on one- or
two-dimensional gels and immunolocalization in cultured cells., Biochem.
J. 306, 211-215.
Walsh, M. P. (2000) Calponin - knocked
out but not down! J. Physiol. 529.3, 517.
Wills, F. L., McCubbin, W. D. & Kay, C. M.
(1993) Characterization of the smooth muscle calponin and calmodulin
complex., Biochemistry. 32, 2321-2328.
Wills, F. L., McCubbin, W. D. & Kay, C. M.
(1994) Smooth muscle calponin-caltropin interaction: Effect on
biological activity and stability of calponin., Biochemistry. 33,
5562-5569.
Winder, S. J., Allen, B. G., Fraser, E. D.,
Kang, H.-M., Kargacin, G. J. & Walsh, M. P. (1993) Calponin
phosphorylation in vitro and in intact muscle., Biochem.J. 296,
827-836.
Winder, S. J., Sutherland, C. & Walsh, M.
P. (1992) A comparison of the effects of calponin on smooth and skeletal
muscle actomysoin systems in the presence and absence of caldesmon., Biochem.J.
288, 733-739.
Winder, S. J. & Walsh, M. P. (1993)
Calponin: Thin filament-linked regulation of smooth muscle contraction.,
Cellular Signalling. 5, 677-686.
Winder, S. J., Walsh, M. P., Vasulka, C. &
Johnson, J. D. (1993) Calponin-calmodulin interaction: Properties and
effects on smooth and skeletal muscle actin binding and actomyosin
ATPases., Biochemistry. 32, 13327-13333.
|
