|
Originally
isolated from Dictyostelium discoideum (de
Hostos et al, 1991),
and subsequently found in other protists (Tardieux
et al, 1998),
vertebrates including Xenopus (Mishima
& Nishida, 1999),
mice and humans (Okumura
et al, 1998),
(see (de Hostos,
1999) for a recent
review), coronin is a homodimeric c 55kDa actin-binding protein. Coronin is
localized to cell surface projections (sometime appearing as "crowns",
thus the name), and generally appearing in actin -rich regions (de
Hostos et al, 1991).
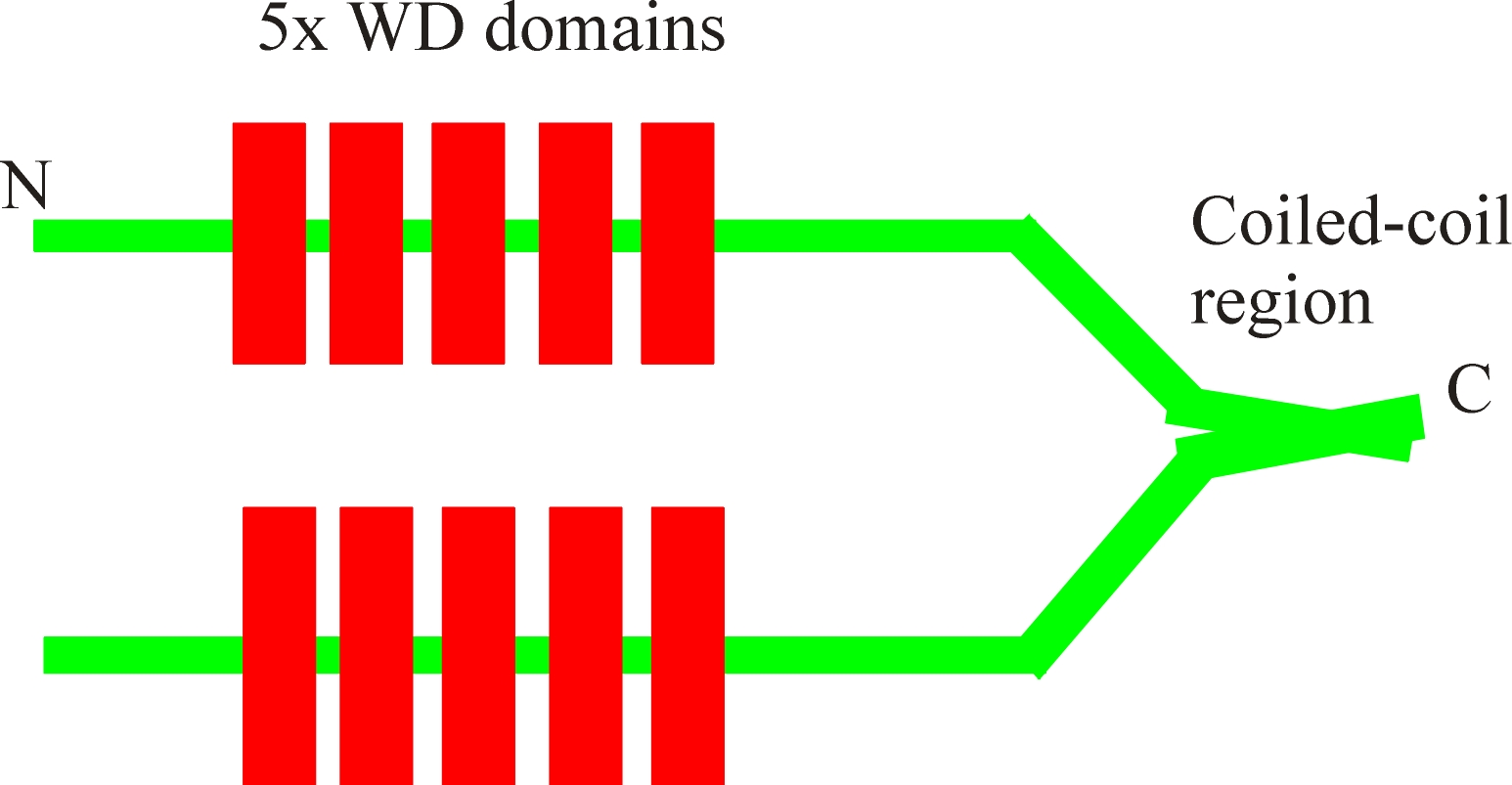
It is now clear that coronin is present in many organisms as a family of
proteins, the main feature of which is the five WD domains. These WD
domains, when present in other protein are known to function as protein:protein
interacting motifs. The five WD domains in coronins form a so called b-propeller
structure within which the actin binding region lies. Exactly where, or
even if the whole structure is required, is not currently known, however the b-propeller
structure itself when expressed alone in bacteria does not bind to actin (Mishima
& Nishida, 1999).
This does not of course mean that this is not the actin binding region as it is
possible that the protein was not correctly folded in the bacteria. A
region of coronin (435-462) has been suggested to be homologous to YpkA, an
actin binding kinase from the bacteria Yersinia suggesting that this may
be an actin binding region (Juris,
et al, 2000).
At the
C-terminal, a coiled-coil region self associated to form the coronin dimer.
This dimer can therefore bind two filaments leading to their gelating and
bundling the actin filaments.
It has been pointed out that emergence of coronin as an actin binding
protein was unlike the many other Dictyostelium ABPs. Typically,
ABPs have been isolated biochemically on the basis of their effects on the
viscosity of exogenously added actin and this activity used as a guide for
purification to homogeneity. After purification either antibodies or limited
peptide sequence information permitted the isolation of cDNAs. Coronin
instead was isolated from a contracted actomyosin pellet and subsequently found
to bind actin. However, when it became possible to knock out these genes from
the haploid organism (see Dictyostelium ABPs), there was some degree of disappointment
as many of the ABPs with strong actin-binding activity produced very little
phenotype. The phenotype of coronin-null cells however, was very obvious, as the
resulting cells were reduced in locomotion and their ability to divide was also
markedly inhibited.
Coronin-null cells are not only defective in locomotion, but grow more slowly,
possibly as a consequence of inhibition of pinocytosis, phagocytosis (Hacker
et al, 1997)
and more certainly by inhibition of cytokinesis (de
Hostos et al, 1993).
In vertebrate cells too coronin is seen to localize to the lamellopodia and when
disrupted by the overexpression with truncated forms inhibits cell spreading and
locomotion (Mishima
& Nishida, 1999),
and the protein appears to be involved in the actin-rich "tails" of Listeria
infected cells (David
et al, 1998).
The function of coronin in cells has been vividly displayed by GFP -coronin
fusion proteins that permit visualization of the protein in living cells (Gerisch
et al, 1995;
also see the links after the references).
In neutrophils the actin cytoskeleton seems to have a role in the regulation of
the NADPH oxidase enzyme assembly around the phagolysosome membrane and there
are indications that coronin is involved in this process (Grogan
et al, 1997).
Other
Related Web-sites:-
References
David,
V., E. Gouin, et al. (1998). “Identification of cofilin, coronin, Rac and capZ
in actin tails using a Listeria
affinity approach.” J.Cell
Sci. 111, 2877-2884.
de Hostas, E. L., B. Bradtke, et al. (1991). "Coronin,
an actin binding protein of Dictyostelium discoideum localized to cell
surface projections, has sequence similarities to G protein b
subunits." EMBO J. 10, 4097-4104.
de Hostos, E. L. (1999). "The coronin family of
actin-associated proteins." Trends Cell Biol. 9, 345-350.
de Hostos, E. L., C. Rehfuess, et al. (1993). "Dictyostelium
mutants lacking the cytoskeletal protein coronin are defective in cytokinesis
and cell motility." J. Cell Biol. 120(1), 163-173.
Gerisch, G., Albrecht, R., Heizer, C., Hodgkinson, S.
& Maniak, M, (1995). "The leading edge of Dictyostelium cells
monitored using a green fluorescent protein-coronin fusion protein" Curr.Biol.
5, 1280-1285.
Grogan, A., Reeves, E., Keep, N, Wientjes, F., Totty,
N.F., Burlingame, A.L., Hsuan, J.J. & Segal, A.W. (1997). "Cytosolic
phox proteins interact with and regulate the assembly of coronin in neutrophils."
J.Cell Sci. 110, 3071-3081.
Hacker, U., Albrecht, R. & Maniak, M. (1997).
"Fluid-phase uptake by macropinocytosis in Dictyostelium." J.Cell
Sci. 110, 105-112.
Juris,
S.J., Rudolph, A.E., Huddler, D., Orth, K., & Dixon, J.E. (2000). "A
distinctive role for the Yersinia protein kinase: Actin binding, kinase
activation, and cytoskeleton disruption." PNAS 97(17);
9431-9436.
Mishima, M. & Nishida, E. (1999). "Coronin
localizes to leading edges and is involved in cell spreading and lamellipodium
extension in vertebrate cells. J.Cell Sci. 112, 2833-2842.
Okumura, M., Kung, C., Wong, S., Rodgers, M. &
Thomas, M.L. (1998). " Definition of a family of coronin-related proteins
conserved between humans and mice: close genetic linkage between coronin-2 and
CD45-associated protein." DNA Cell Biology. 17, 779-787.
Tardieux, I., Liu, X., Poupel, O., Parzy, D., Dehoux,
P. & Langsley, G. (1998). "A Plasmodium falciparum novel gene
encoding a coronin-like protein which associates with actin filaments." FEBS
letters 441, 251-256.
|
