BB1 |
|
A very small
marine amoeboflagellate that alternates between a limax and flabellate type morphology.
Isolated from seawater off Bournemounth Beach in April 2000. At least two genera of
amoeba are known to alternate between limax and flabellate modes, Rhizamoeba and
Learamoeba.
The life cycle of BB1 is typical of the Vahlkampfiidia as is the eruptive habit of the
pseudopodia during the very active locomotion (in limax mode). |
|
|
|
|
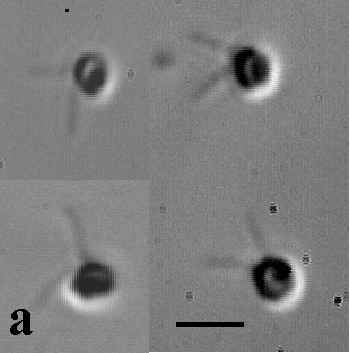
| Figure 1a.
The flagellate stage of BB1, stained and fixed in Lugol's Iodine. Bar = 4mm. The flagellate body is spherical and the flagella
2.5 - 3.0
mm in length. |
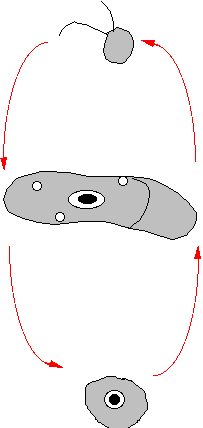
Figure 2b. The proposed life cycle of BB1 it is not yet
clear whither or not the flagellate stage can multiply. Top, flagellate,
middle amoeba, bottom cyst. |
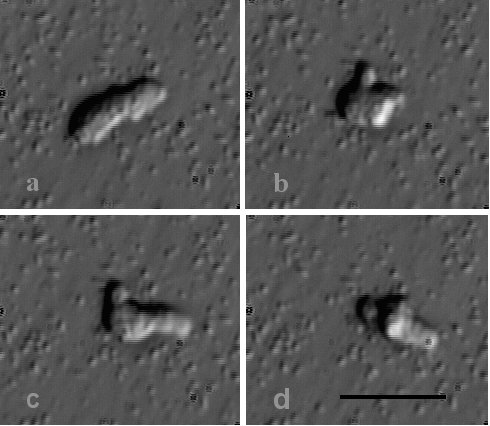
Figure 1c.
The amoeda stage. The same individual is imaged at 5 second intervals to
demonstrate motility and eruptive pseudopd formation. The amoebae adhere poorly to
glass often pivotting about the uroid. bar = 10mm. |
|
Adhesion of BB1
to both glass and tissue culture plastic is very weak, limiting the speed of locomotion,
however on agar, this amoebae moves rapidly. Figure 1c shows an individual adhering
by the uroid and extending pseudopods but getting nowhere fast! Rogerson (Rogerson 1993) has drawn attention to the
fact that amoebae smaller than 10mm are extremely under
represented in the amoeba literature and yet are very abundant in soils, sediments,
freshwater and seawater. The small size of BB1 and the other dimorphamoebae may
allow the amoebae to penetrate very fine grain sediments such as the very productive mud
flats around coastal fringes especially in mangrove swamps and estuaries. Pore space
in sediments is proposed to be a limiting factor in bactivore activity in salt marshes (Elliot & Bamforth, 1975). Despite
the fact that in the water column above mangrove swamp sediments is extremely rich in
amoeba, the sediments themselves are reported to be sparsely populated by amoeba.
Amoebae were described as being rare in salt marsh sediments too (Elliot & Bamforth, 1975). These
reports of low amoebae numbers could be due to the fact that the studies did not set out
deliberately to search for amoebae (they would be very difficult to see by direct
microscopy) in sediments. Another factor could be that the predominant amoeba in the
sediments may be of the small. type described here. The dimorphamoeba may be an
important group in bacterial turnover in aerobic mudflat sediments, while anaerobic
cousins may be important in deeper sediments. Recently, the micro-aerophilic
amoebo-flagellate Psalteriomonas lanterna has been placed very close to the
Vahlkampfiidae despite the fact that this amoeba is amitochondriate having hydrogenosomes
instead (Weekers et al, 1997),
indicating the possible existence of similar amoeba is marine anoxic sediments. This group
are too small to differentiate microscopically and so this requires
biochemical and
genetic approaches (see Dimorphamoeba).
|
| References:-
Elliott, P. B. & Bamforth, S.S. (1975). "Interstitial
protozoa and algae of Louisiana salt marshes." J.Protozool. 22(4):
514-519.
Rogerson, A. (1993). "Parvamoeba rugata n.g., n.sp.,
(Gymnamoebia, thecamoebidae): An exceptionally small marine naked amoeba." Eur.J.Protozool.
29: 446-452
Weekers, P. H. H., Kleyn, J., & Vogels, G.D. (1997).
"Phylogenetic position of Psalteriomonas lanterna deduced from SSU rDNA
sequence." J.Euk.Microbiol. 44: 467-470.
|
|
|
|
