|
Isolation
of Amoebae |
|
Page updated 23/8/02 |
Amoeba
are essentially everywhere and can be isolated from almost any kind of
sample; soil, freshwater or salt water. Most methods for isolating
amoeba are based on the "walk out" method in which a
mono-layer of bacteria (usually E.coli) is deposited on an agar
plate, and any amoeba present inoculum crawl as they consume the
bacteria, to produce characteristic haloes of growth. Of course,
just as 95% of bacteria cannot be easily cultured, by simple inoculative
methods, so we can be certain that a proportion of the amoeba initially
present cannot be cultured by this simple method and are rapidly
overgrown by the usual amoebal "weeds"! This makes it
very important to consider what type of organism we would wish to
culture.
The source of sample is a very important influence over which type of
amoeba predominates in the culture. For example, if one is
interested in obtaining Naegleria, then the best source would be
freshwater. If Acanthamoeba are desired then soil is
the best bet especially if the soil is rich in organic content.
Flattened or flabellate amoeba (Vannella, Platyamoeba etc) are
most likely to be found in marine samples
Several different approaches can be taken
for the isolation of amoebae depending on which type of amoebae is
required. The main determinant in the choice of method is size.
Three methods are outlined below in order of the size of the amoebae the
isolator is interested in. |
|
Small Amoebae
(2-30 microns spherical diameter) Acanthamoeba, Naegleria,
Vannella
This basis of this method was published in the late 50's (Harrison,
1957; Neff,
1958). If amoeba are being sampled
from water, this is poured over a petri dish in which 2% agar has been
deposited. For freshwater, the 2% agar should be in Neff's saline (see
below). If the water is seawater, then the plates should contain
2% agar in 75% seawater (for no clear reason many amoeba from fully
marine environments do better in this than 100% sewater (Page,
19). If soils/sediments are being sampled a small amount of the
material should be deposited on the plates with suitable water to
cover. Amoebae should be given a few days to gather on the plates,
after which, small cubes (A,
figure 1) should be excised and placed upside down on fresh plate into
which bacteria (usually E.coli) have been spread and allowed to
dry. Ciliates and other non crawling protists together with
metazoans are left under this block (sometimes nematodes wriggle out if
the plates are too wet), while amoebae are free to crawl out from under
the block consuming the bacteria and growing as they move out.
After a few days characteristic growth rings appear on the surface
(Figure 1, left). Amoeba are often very numerous at the leading edge (red),
but for some reason Acanthamoeba does not do this much. Being
this concentrated region, amoebae are present as small groups and
individuals, but without much bacteria on which to feed (blue),
further back toward the original block, amoebae especially from soil and
freshwater will have formed cysts as a result of descication and lack of
bacteria (green).
The cysts often flocculate. The amoeba from this culture turned out to
be Naegleria gruberi. |
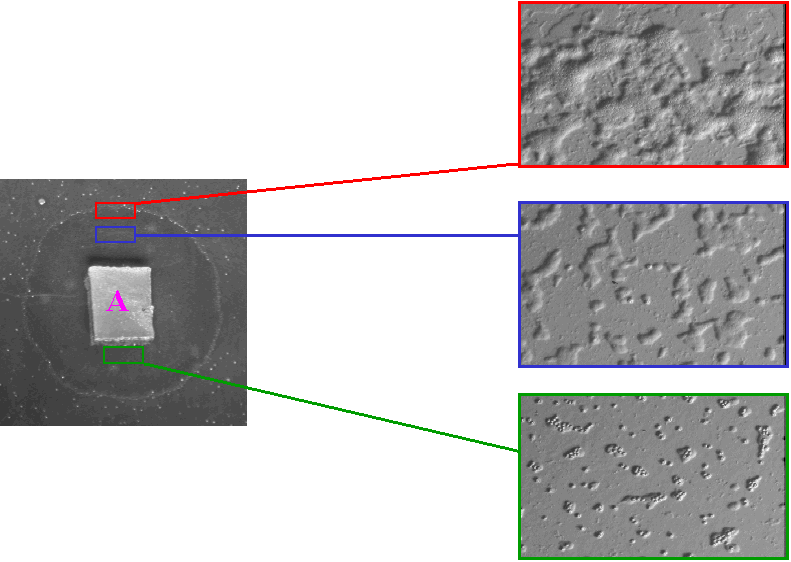
Figure 1. Culturing of amoeba by the
"walk out" method. |
Small blocks of agar from this plate
should then be excised and the amoebae shaken off the block by swirling
in media (such as sterile Neff's or 75% seawater), and deposited on
fresh agar plates. Small blocks from these plates are now excised
and inverted on fresh bacterial lawn plates and the process
repeated. By now the culture should be clonal, but the only way to
be sure is to isolate a single amoeba by carefully excising a block on
which only one amoeba is resting.
Axenic culture
Many of these small amoeba can be cultured axenically, that is without
any other organism present. This is often desirable if the amoeba
is to be characterised at the molecular level or grown in large amounts
as a source of a particular molecule. The walk out method can be
used for axenification by creating lawns of heat killed (autoclaved
bacteria) and the use of antibiotics (such as penicillin) in the
agar. Blocks of agar containigndead bacteria and living amoeba can
then be transferred to sterile axenic media. (see Culturing
amoebae). |
| WARNING.As
some of this group are known to be pathogenic in humans and animals,
care should be taken with the resulting cultures. Workers wearing
contact lenses should be especially cautious with Acanthamoeba
strains as some cause keratitis, a potentially sight threatening
condition. The pathogenicity problem can be reduced by discarding any
cultures in which amoeba can grow at 37oC. |
|
Medium Amoebae (30-150 microns) Mayorella,
Saccamoeba,
The walk out method described above can be used for these larger
species, but the small amoeba tend to out grow them, obscuring there
presence and out competing them due to their more limited requirement
for surface water films. Instead the following dilution method may
be better. Pond, sea or soil is plated out on suitable agar plates
as above and amoeba are allowed to settle. It is also worth doing
without the agar. A single interesting larger amoeba can be
scooped up by the careful use of a pipette and this transferred to a
well in a 24 well plate. Let everything settle down and then look for
the successfully transferred amoeba. If it is there and there are
no other similarly sized amoeba present, growth can be encouraged by
adding food organisms such as bacteria or Tetrahymena.
Larger amoeba tend to require more than just bacteria to grow, many need
ciliates or even other amoeba. |
| Giant Amoebae (150-4000 microns) Amoeba,
Chaos, Pelomyxa
Giant amoeba such as those of the genera Amoeba,
Chaos and Pelomyxa are not the most numerous and can
be difficult to find. They are to be found most often on the muddy
surfaces of well established ponds and lake sediment. One method to collect them is
to invert a clear glass jar into the water so that air is retained until
the lip reaches the silty pond bottom. Air is then let out so that
the inrushing water sucks in the top layer of silt. Move the
vessel (jar) around to suck up a large surface of the pond bottom.
Tall jars are particularly useful as the next step is to settle the
contents of the jar in diffuse (not direct sunlight) with the bottom of
the jar covered in foil. Amoeba and Chaos are
phototactic (I don't know if Peloxyma is). The amoeba will
concentrate on the surface of the "pond" within the jar.
Once the amoeba is discovered it can be
transferred into a petri dish with a pipette and filtered pond water
added. Boil a few grains of rice let them cool and transfer one to
the petri dish, organisms that will have inevitably come along with the
amoeba will grow and provide food. Alternatively, ciliates such as
Tetrahymena can be grown and fed to the amoeba.
The amoeba to the right ( A.
proteus) was isolated from the pond above. |

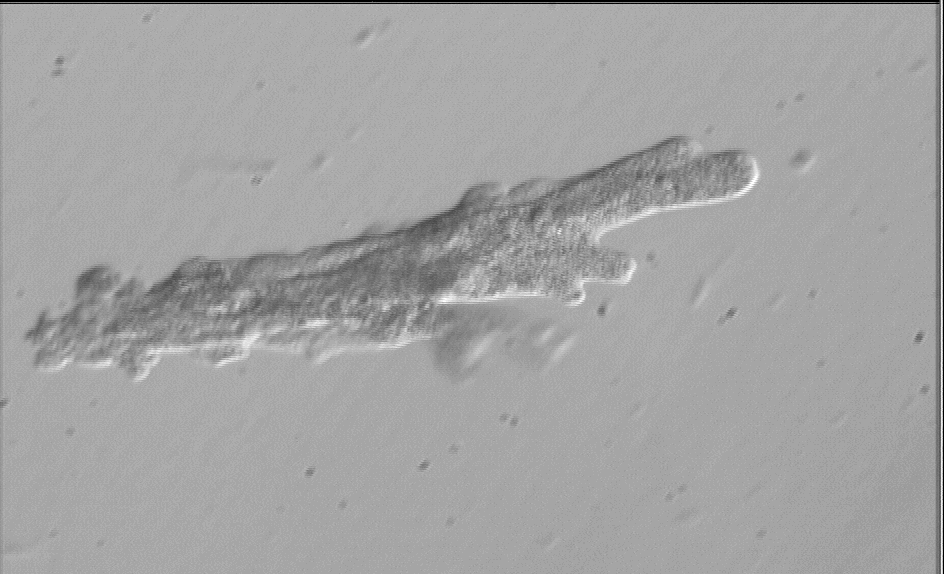 Amoeba
proteus Amoeba
proteus
|
| References:-
Harrison, D. (1957) A technique for
obtaining clone cultures of soil amoebae in sterile liquid medium. Nature.
180, 1301-1302.
Neff, R. J.
(1958) Mechanisms of purifying amoebae by mirgation on agar
surfaces. J.Protozool. 5, 226-231. |
|
